Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
Chlorine dioxide was discovered in 1811 by Sir Humphry Davy and since the mid-20th century, it has been widely used in the paper industry as a bleach and for the treatment of drinking water.
- chlorine dioxide
- toxicity
- “miracle mineral solution”
- human consumption
1. Introduction
Chlorine dioxide (ClO2) is classified by the World Health Organization (WHO) as a safe and effective fourth generation, broad-spectrum, class A1 disinfectant [2,3]. It is used to purify drinking water without creating harmful concentrations of disinfection by-products [4]. The properties of ClO2 result from one-electron transfer reactions, so it is considered a strong oxidizing agent [5] and, unlike chlorine, does not tend to react with organic materials to form chlorinated species or with ammonia to form chloramine. Chlorine dioxide is an important biocide and bleach and is used as an alternative to chlorine in the purification and disinfection of drinking water [6]. ClO2 is used in 8.1% of drinking water treatment plants in the USA and 32.8% of those in China [7,8], and in some European countries [9], it is used in paper bleaching, sterilization of medical devices, and disinfection of foodstuffs [10]. According to the EPA, ClO2 is used “in public water-treatment facilities, to make water safe for drinking.” When chlorine dioxide is added to drinking water, it helps to destroy bacteria, viruses, and some types of parasites that can make people sick, such as Cryptosporidium parvum and Giardia lamblia.
Its main advantage over chlorine is that it reduces the formation of harmful organochlorine compounds [11,12,13,14,15,16,17]. ClO2 is beneficial in minimizing the formation of trihalomethanes; however, ClO2 is converted to ClO2− and ClO3−, which can cause hemolytic anemia and other health effects. The Environmental Protection Agency (EPA) has set the maximum concentration in drinking water at 0.8 milligrams per liter (mg/L) for chlorine dioxide and 1.0 mg/L for chlorite ions [18]. Some of its industrial applications are listed in Figure 1.
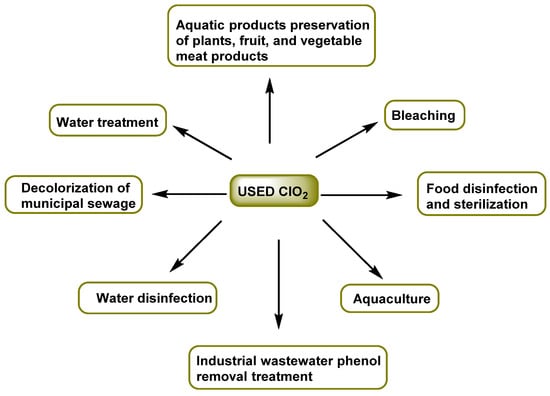
Figure 1. Applications of chlorine dioxide.
Chlorine dioxide is a compound that differs from elemental chlorine, both in its chemical structure and in its behavior [19]. An important characteristic is its high solubility in water, especially in cold water. Chlorine dioxide is about 10 times more soluble in water than chlorine.
2. Physicochemical Properties of ClO2
ClO2 is a yellowish-green gas and has a pungent odor, like Cl2, with a boiling point of 11 °C, a melting point of −59 °C, a density of 1.64 g mL−1 (liquid) at 0 °C, a water solubility of 3 g L−1 at 25 °C, and a pKa = 3. ClO2 is very soluble in water and does not hydrolyze, remaining in solution as a dissolved gas [20]. Solutions of ClO2 in water are stable when protected from light and kept at room temperature or below, well-sealed, and slightly acidified (pH = 6). The ultraviolet absorption spectrum of ClO2 solutions is broadband with a peak at 359 nm and a molar extinction coefficient of 1250 M−1 cm−1. ClO2 has a relatively short half-life and is highly volatile and explosive at concentrations > 10% in air [21]. Chlorine dioxide may not be compressed, stored, or transported under pressure and must be manufactured at the place of consumption.
ClO2 is a neutral monomeric free radical with a dipole moment of 1.792 Debye [22]. From the microwave spectra of gas-phase chlorine dioxide, the chlorine-oxygen distance is found to be approximately 0.147 nm and electron diffraction indicates 0.149 nm. This chlorine-oxygen distance is approximately that of an average chlorine-oxygen double bond. Studies on the geometry of ClO2 established that the bond distance between the Cl atom and the O atom is smaller compared to the bond in chlorine monoxide (ClO). These results explain and justify the representation of the double bond between these two atoms, as well as showing that resonance structures satisfactorily explain the unpaired electron of the chlorine atom. ClO2 has a molecular geometry with an oxygen-chlorine-oxygen bond angle of 117.6°, as shown in Figure 2. In its ground state, although the unpaired electron is shared between the two oxygen atoms and the chlorine atom, most of the electron density resides mainly on either oxygen atom.
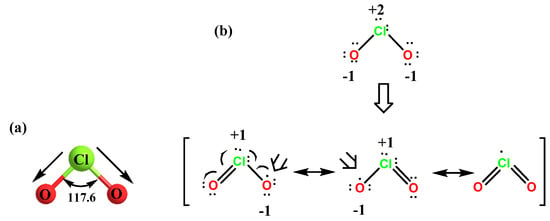
Figure 2. (a) The molecular structure of ClO2. (b) The Lewis structure of ClO2.
It has an odd number of valence electrons (it is a paramagnetic radical), and its electronic structure has long puzzled chemists, because none of the possible Lewis structures are satisfactory. In 1933, Brockway proposed a structure involving a three-electron bond [23]; Linus Pauling later developed this idea and proposed two possible resonant structures involving a double bond on the one hand and a single bond with a three-electron bond on the other [24].
The electronegativity of the two oxygen atoms is large enough to eliminate the electron density of the chlorine atom and gives chlorine a partial positive charge, Figure 2.
3. Generation of Chlorine Dioxide
Chlorine dioxide is a widely used disinfectant as an alternative to chlorine, due to its effectiveness in pathogen inactivation and low production of halogenated organic by-products of disinfection. However, during the generation of ClO2, chlorine is inevitably introduced into the ClO2 solution obtained as an impurity. The presence of chlorine in chlorine dioxide may affect the formation and toxicity of disinfection by-products as well as the disinfection efficiency.
There are different methods for the preparation of chlorine dioxide [16], depending on the amount required, the number of by-products that can be tolerated, and whether the gas is required in solution or in gaseous form, Figure 3.
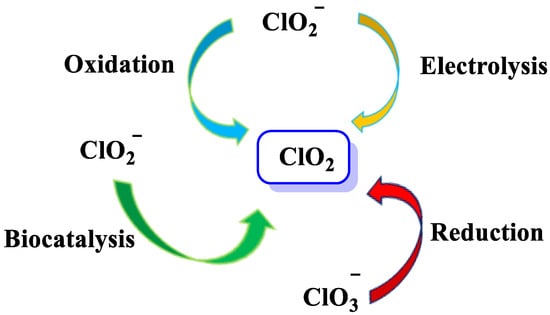
Figure 3. ClO2 preparation methods.
3.1. From Chlorite Ions
ClO2 is generated from chlorite ions using chemicals, electrochemicals, and biocatalysts, and from the reaction of chlorite with chlorine gas Cl2 or hydrochloric acid (HCl) [25,26,27], as shown in Figure 4.
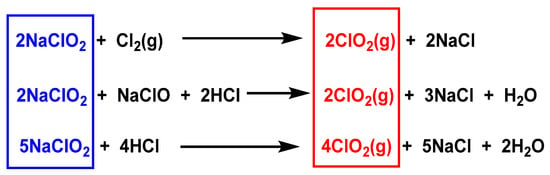
Figure 4. Preparation of chlorine dioxide from sodium chlorite.
The methods described in Figure 4 have major disadvantages due to the production of high amounts of chlorides, which can be avoided by replacing hydrochloric with sulfuric acid, although in such cases, the processes become less efficient. These methods involve concentrated acids and/or externally added oxidants such as Cl2, OCl−, and H2O2.
Another way to generate ClO2 from chlorite by one-electron transfer is by electrochemical means [28], but this procedure requires a considerable input of electrical energy. An electrochemical method using mixed metal oxide MMO electrodes in the presence of chlorite and boron-doped diamond BDD anodes to promote the evolution of chlorine species was also studied [29,30]. Another possibility is to start from an undoped solution of sodium chlorite and a mixture of sodium chloride in an undivided electrochemical cell with a constant current, Ti/IrO2 anode, and Ti/Pt cathode [31,32].
To oxidize chlorite to ClO2, catalysts are based on manganese or iron porphyrin complexes. In these systems, chlorite dismutation is initiated through the oxidation of Mn(II or III) or Fe(III) by chlorite ions to produce hypochlorite ions and high-valent Mn and Fe(IV or V). Both oxidation states, IV and V, oxidize chlorite directly to ClO2, although complete conversion of chlorite to ClO2 was achieved in water using water-soluble Fe or Mn porphyrins. The synthesis of these ligands and catalysts is very expensive. A catalytic process has also been developed using a manganese porphyrin catalyst, tetra-kis-5,10,15,20-(N,N-dimethylimidazolium) porphyrinatomanganese(III), which is soluble in water and catalyzes the formation of chlorine dioxide from chlorite at room temperature and pH = 5 [33,34,35,36].
3.2. From Sodium Chlorate
Currently, the most widely used method to produce chlorine dioxide is the reduction method, by reacting sodium chlorate in a concentrated acid solution with reducing agents such as sulfur dioxide, methanol, oxalic acid, hydrogen peroxide, hydrochloric acid, or sodium chloride. With hydrochloric acid, the chlorine content is high, the purity of the chlorine dioxide is low, and the contamination is severe [37,38].
The sulfur dioxide method has the disadvantage of SO2 [38], with side reactions and low efficiency, which limits its application. The methanol process is currently the most widely used method for chlorine dioxide production in new-build plants worldwide [37]. The chlorine dioxide obtained is of high purity, but this method requires high acidity, and the reactor needs materials with excellent corrosion resistance.
In the chlorate reduction method, hydrogen peroxide advantageously replaces the other reagents, the process is more environmentally friendly, and the main by-product formed is oxygen, Figure 5.
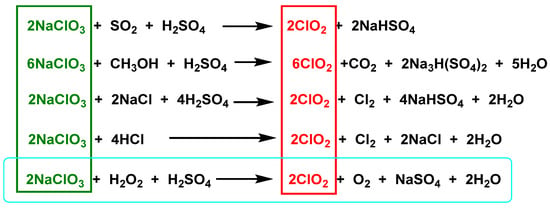
Figure 5. Preparation of chlorine dioxide from sodium chlorate.
The reaction between commercial solutions of chlorate and H2O2 results in the formation of ClO2 [38]. The reaction is very reproducible and stoichiometric. It is very important that the reaction mixture is not depleted of chlorate to avoid further reduction of ClO2. Once ClO2 is formed, the reduction of the chlorinated species continues, leading to the formation of other species, such as chlorite, Figure 6.
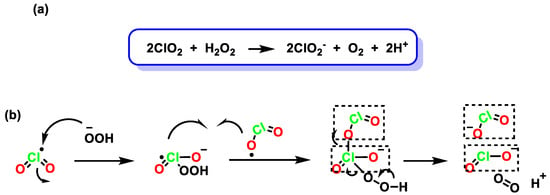
Figure 6. (a) Reduction of chlorine dioxide with hydrogen peroxide. (b) Reduction mechanism of chlorine dioxide with hydrogen peroxide.
When large quantities of chlorine dioxide are needed, sodium chlorate is used as a raw material, and this method has traditionally been used in the pulp and paper industries. The conditions for producing ClO2 from sodium chlorite can be better controlled than those for sodium chlorate, but chlorite is more expensive and unstable, and therefore, from an industrial point of view, sodium chlorate is a more suitable feedstock [39].
This entry is adapted from the peer-reviewed paper 10.3390/ijms232415660
This entry is offline, you can click here to edit this entry!
