Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Catalytic ozonation belongs to the Advanced Oxidation Processes (AOPs) because it is expected to enhance the production of hydroxyl radicals (considered as strongly oxidant agents).
- catalytic ozonation
- micropollutants
- Advanced Oxidation Processes
1. Introduction
Chemical oxidation processes are used as common alternatives of biological treatment processes, especially for the removal of non-biodegradable compounds. The aim of chemical oxidation processes is the faster and better mineralization of emerging contaminants; ozonation is among these processes. However, the basic disadvantage of single ozonation is that it rarely leads to total mineralization. Instead, saturated organic compounds, such as aldehydes or short-chain carboxylic acids, can be formed by the partial oxidation of the original substances [1]. In addition, ozonation can be characterized as a relatively costly process in comparison with other alternative treatment methods. However, it presents specific advantages, as well as certain disadvantages, compared to the other available Advanced Oxidation Processes (AOPs) dealing with the removal of micropollutants (e.g., Fenton) [2]. These disadvantages may be overcome by adding an appropriate catalyst, and the modified process is then called “catalytic ozonation”. The main advantages of catalytic ozonation over the conventional process are relevant with more efficient ozone consumption and faster process throughput due to the higher rates of mineralization and higher removal efficiency of oxidized compounds [3].
Catalytic ozonation belongs to the Advanced Oxidation Processes (AOPs) because it is expected to enhance the production of hydroxyl radicals (considered as strongly oxidant agents). According to the specific type of catalyst added to the oxidation system, the catalytic ozonation can be divided into homogeneous and heterogeneous catalytic ozonation when transition metal ions or solid materials are used as catalysts, respectively [4]. Heterogeneous catalytic ozonation is advantageous over homogenous catalytic ozonation due to the easier separation of the catalyst (and its potential reuse) from the aqueous solution in which the oxidation reaction takes place [5].
The frequency of the studies that have been published in the field of heterogeneous catalytic ozonation over the last 20 years (2002–2022) with the use of different solid catalysts is shown in Figure 1. Note that most of these studies are based on batch experiments. The relevant literature was collected from Science Direct, Scopus, MDPI, and PubMed databases using relevant keywords, such as heterogeneous catalytic ozonation, advanced oxidation processes, catalysts, micropollutants, etc. From Figure 1, it becomes clear that heterogeneous catalytic ozonation is a relatively recent investigated process, which began to be studied systematically during the last ten years. Of the approximately 30,000 studies, concerning this process, 24,000 were published from 2010 onwards. Most of these studies are related to the production of an efficient catalyst or to the modification of commercial materials, usually by depositing proper metals into their structure, whereas a lower number of studies refer to the investigation of the reaction mechanism(s).
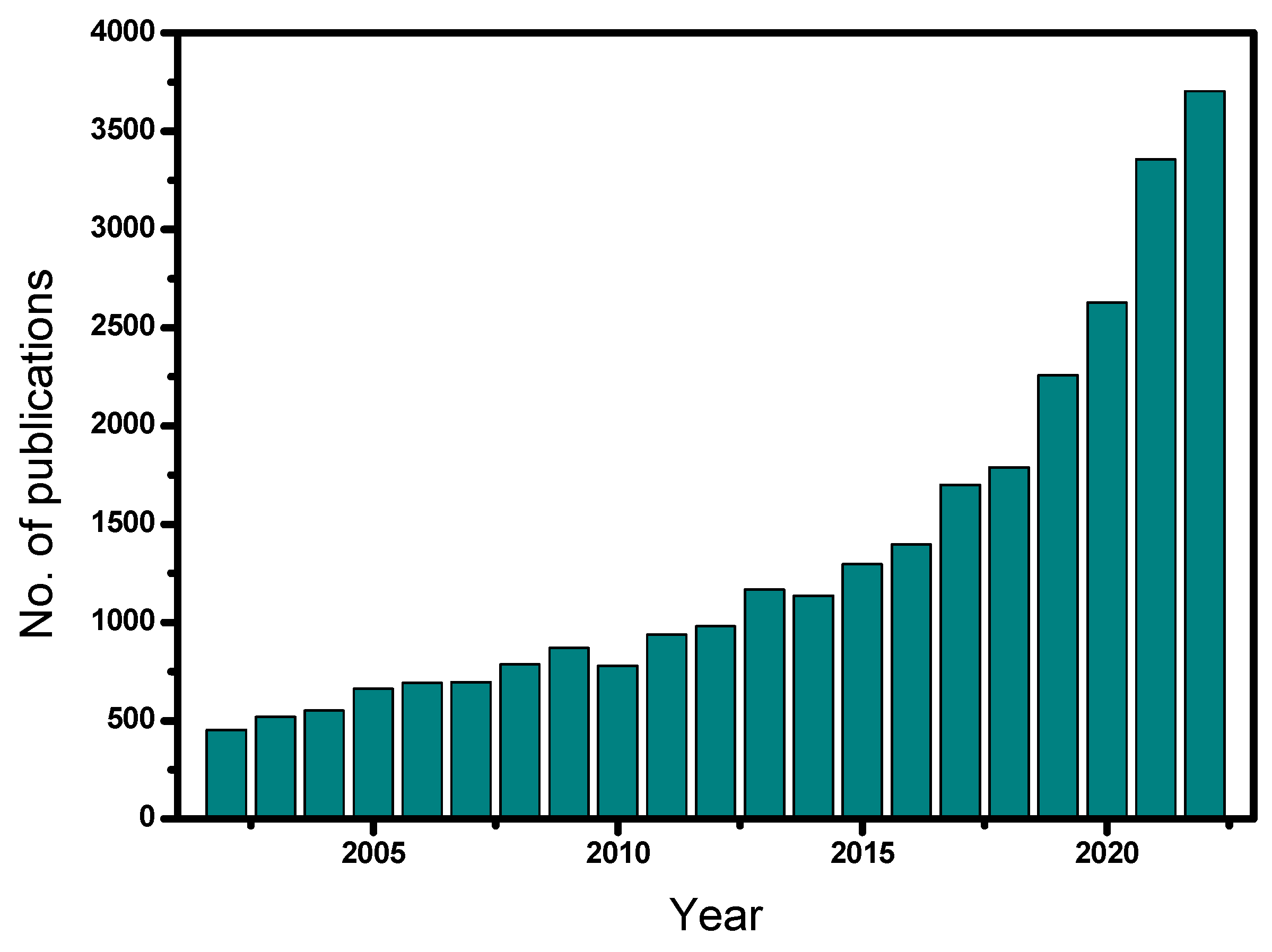
Figure 1. Published scientific studies on heterogeneous catalytic ozonation for the period 2002–2022.
In heterogeneous catalytic ozonation, the applied catalyst is in solid form and the reaction takes place either in the bulk (aqueous) solution or onto its surface. When the combination of ozone with a solid material is considered as a catalytic ozonation process, then the efficiency of ozonation in the presence of the solid must be greater than the sum of the removals caused by the adsorption of the pollutant and by the effect of single ozonation under the same pH value [6]. However, the highest catalytic activity is noticed when the difference between single and catalytic ozonation is the highest. The key to heterogeneous catalytic ozonation efficiency is to find the appropriate (solid) material that can act as an effective catalyst [7]. The catalytic effect takes place when one of the following conditions is met [4]:
-
Ozone is adsorbed on the surface of the catalyst.
-
The organic molecule is adsorbed on the surface of the catalyst.
-
Ozone and the organic molecule are both adsorbed on the surface of the catalyst and then interact.
Radical [8] and non-radical [9][10] mechanisms are also reported, although the most common is the radical mechanism. In nearly all cases occurring through the application of radical mechanisms, the predominant species are the hydroxyl radicals; however, there are certain studies in which the hydroxyl radicals are not responsible for the removal of micropollutants, while the superoxide radicals [11][12] or the single oxygen atoms [11][13] are the main oxidizing species. Superoxide radicals are less powerful oxidizing agents than the hydroxyl radicals. However, they can form singlet oxygen atoms, which are more powerful and selective oxidizing species, such as hydroxyl-free radicals and hydrogen peroxide [14]. More information about the mechanism of these radical species produced during the AOPs processes can be found in Gottschalk et al. [15] and Rayroth et al. [16]. The non-radical mechanism can take place via two main specific routes: (1) surface complexes formed by the adsorption of ozone and pollutant on the solid catalyst surface and (2) adsorbed oxygen species onto the solid surface by the dissociation of O3 that are in contact with the catalyst active sites [13]. Examples of these two main mechanisms are presented in Figure 2.
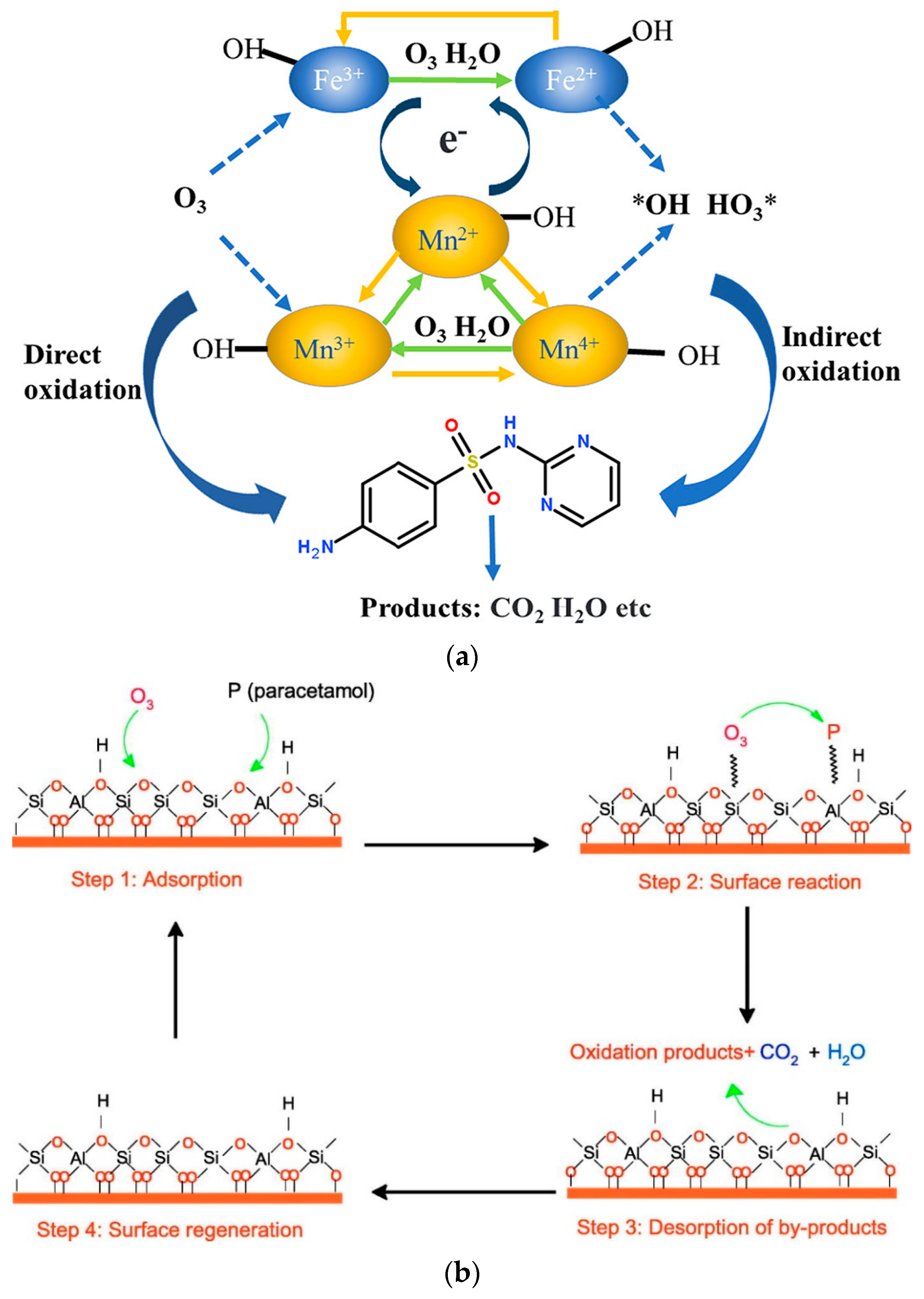
Recently, Inchaurrondo & Font [17] published a review regarding the use of natural (catalytic) materials in the ozonation of organic pollutants. This research concludes that despite the large number of studies concerning the catalytic ozonation mechanism(s), there is still a lack of understanding. However, this is not something new; already in 2013, Nawrocki published a relevant paper on the controversial issues observed between the heterogeneous catalytic ozonation literature studies [4], reporting seven relevant cases and in particular: (1) lack of proper pH control, (2) adsorption of substrate onto the catalyst surface, (3) adsorption of ozonation products, (4) realistic catalyst-organic substrate ratio, (5) purity of the catalyst, (6) “one run” catalyst, and (7) natural water conditions. Overall, this research summarizes the studies on heterogeneous catalytic ozonation at that time, attempting to limit the aforementioned controversial factors while trying to reach valid conclusions. The literature studies included in the current research use mostly stable catalysts (except for some specific cases, as mentioned in the text) in realistic ratios with the examined organic compounds and under stable pH values. The evaluation of these studies used as main indicator their efficiency with respect to adsorption, single ozonation, and/or catalytic ozonation. Furthermore, the present research introduces two more factors that are considered as most important for the evaluation and comparison of the heterogeneous catalytic ozonation processes, i.e., (1) the pH value of (aqueous) medium and (2) the (small) initial concentration of examined organic compounds. Moreover, the studies presented in this research are based on simulated experimental conditions, as the relevant ones conducted with natural water conditions are rarely reported.
The pH value is a very important factor for ozonation processes because it can highly affect the decomposition of ozone, as well as the surface properties of used solid materials/catalysts (e.g., the point-of-zero charge/PZC) and the charge of the examined organic compounds. Additionally, it affects the electrostatic interactions between the pollutant and the catalyst surface [8][18]. As Figure 3 shows, the increase of pH value can lead to increased ozone decomposition, which subsequently leads to the production of more hydroxyl radicals. The hydroxyl radicals are more powerful oxidizing agents than the ozone and, therefore, when they predominate in an oxidation reaction, the process efficiency becomes higher. In contrast, the stability of ozone molecules at the acidic pH values is higher and, hence, it is more difficult to be decomposed. However, the effect provoked by the pH value mainly depends on the specific type of applied AOP. As Aziz et al. [19] showed, the single ozonation process under acidic pH values presents lower efficiency with respect to the removal of micropollutants, while the Fenton process is more effective for these pH values. In the present research, the removal of micropollutants from common aqueous matrixes is evaluated and, therefore, the pH values of respective studies are in the range of those usually encountered in natural waters (i.e., 6–8). These observations can affect not only the efficiency, but also the mechanism of the catalytic ozonation process. More information about the effect of pH value on ozone decomposition and the subsequent production of hydroxyl radicals can be found in the aforementioned reference [19].
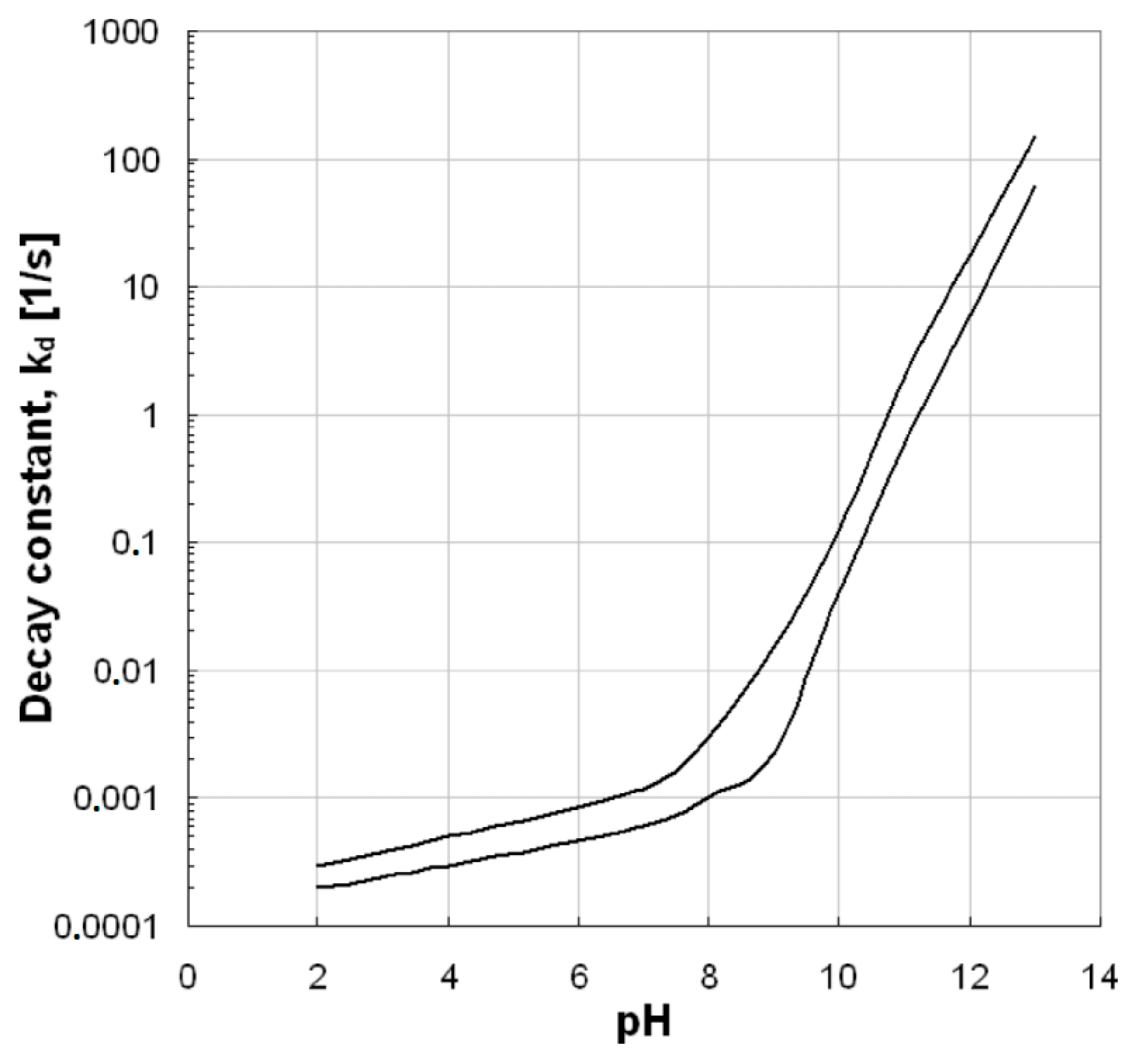
Figure 3. Ozone decomposition constant as a function of pH value [20].
The pH of the (aqueous) matrix solution should be constant throughout the oxidation reaction in order to properly evaluate (and compare) the ozonation process with other alternatives. Su et al. [21] used Fe3O4@SiO2@MgO material as a catalyst and compared its performance with the efficiency of separate FeO4@SiO2 and Fe3O4 solid compounds. The three catalysts have PZC values equal to 9.6, 7.16, and 7.01, respectively. The pH of the solution was not buffered, but it was adjusted before the initiation of the oxidation reaction. They observed that the optimal catalyst was Fe3O4@SiO2@MgO. However, the presence of MgO in its structure possibly could increase the solution pH (towards alkaline values), unlike the other two catalysts, thus, promoting the decomposition of ozone and the higher production of hydroxyl radicals. Nevertheless, under real (not simulated) conditions, the solution pH can present certain buffering capacity, and, therefore, its pH value is not expected to change significantly by the addition of solid catalysts [22]. In this research, the efficiency of the catalytic process by using these three solids cannot be compared.
There are numerous studies that have shown that during catalytic ozonation, the mineralization of pollutants increases, while the removal of pollutants presents lower or equal efficiency, as compared to the application of single ozonation. Moreover, there are certain cases where the catalytic ozonation process proved to be suitable for the reduction of DOC in wastewater, yet the removal of the pollutants content was not considered as sufficient [23][24][25][26]. The micropollutants commonly occur in low concentrations (few μg/L) and are not easily degraded by the presence of ozone molecules, as they can be attacked/oxidized with smaller possibilities. The main by-product of ozone decomposition, i.e., the hydroxyl radicals, are more powerful oxidizing agents than the ozone molecule, but they present shorter lifetime (duration of only some seconds) [27]. These two factors, overall, can reduce the efficiency of catalytic ozonation in terms of micropollutants removal. For example, Liu et al. [26] used the composite material Zn-CNTs for the removal of 4-chloro-3-methyl-phenol. The zero-valent form of zinc (Zn0) is a strong reducing agent, and when it reacts with oxygen, it can produce H2O2, which is expected to enhance synergistically the catalytic ozonation process. The production of H2O2 was even higher for the Zn-CNTs/O3 system because it can be produced both by ozone decomposition and by the reaction between Zn and O2 in the system. However, this catalytic combination showed lower efficiency than the case of single ozonation, probably because the oxygen atoms occupied the most active sites of the catalyst (to produce H2O2) and they were unavailable for the required oxidation purpose. Simultaneously, the competition between the H2O2 and the examined organic compound with the ozone molecules reduced the effect of the Zn presence. However, even though the removal of the pollutant was not improved by the addition of this catalyst, the total mineralization of the studied oxidation system was enhanced.
2. Catalyst Categories
The major categories of solids commonly used as catalysts in the heterogeneous catalytic ozonation process for the degradation of organic pollutants in aquatic solutions are [4]:
-
Metal oxides, such as MnO2, Fe3O4 or Al2O3.
-
Metals, such as Fe, Mn, Co, Cu, and Ce deposited onto different substrates, such as Al2O3, MCM-41 or SBA-15.
-
Minerals, such as zeolites, perovskites, cordierite, and ceramic honeycomb.
-
Carbons, such as AC, GO, and CNTs.
Among them, the category of carbons is the most controversial. There are studies showing that carbons are not stable under the strong oxidizing conditions created by the presence of ozone; thus, they do not present the usually required for long-term stability during the application of ozonation processes. Valdés et al. [28] reported that the exposure of activated carbons to ozone can lead to the modification of their surface properties and to different textural characteristics, where the following equations can possibly take place [29]:
HO− + C* → C*-HO−
C*-HO− + O3 → C*-O3− + HO•
In the 1st reaction (Equation (1)), the hydroxide ions (HO−) are adsorbed onto the surface of solid material, and then (Equation (2)) the ozone reacts with the adsorbed hydroxide ion, producing hydroxyl radicals. These reactions eventually can lead to the further decomposition of C*-O3− product; thus, the carbon adsorption centers are occupied by the hydroxyl ions, reducing their overall adsorption capacity. As a result, although the hydroxyl radicals are produced from these reactions, most of the adsorption centers in the solids (carbons) surface are occupied, and the reaction of ozone (according to Equation (2)) with the substrate is restricted [29]. Razumovskii et al. [30] has also reported that exposure to ozone can decrease the surface reactivity that may protect the surface of carbons from the destructive (oxidation) action of ozone.
Additionally, ozone treatment has as a consequence the transformation of surface alkaline carbon sites towards acidic ones and, thus, the generation of new acidic sites in the system, causing the reduction of the PZC value. Valdes et al. [28] reported that after 120 min of ozone exposure, the PZC of carbon was reduced from 8.8 to 2; the same observations were reported also by other researchers [31][32]. Ozone can affect also the structural characteristics of carbons by reducing their surface area because the pore walls are destroyed by the ozonation of carbons [28][33]. Furthermore, when considering catalytic activity, Sanchez-Polo & Rivera-Utrilla [33] reported that the decrease of alkaline (oxygenated) surface groups has led to the reduction of H2O2 production, thereby decreasing the process efficiency overall.
This entry is adapted from the peer-reviewed paper 10.3390/separations9120413
References
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673.
- Mousset, E.; Loh, W.H.; Lim, W.S.; Jarry, L.; Wang, Z.; Lefebvre, O. Cost Comparison of Advanced Oxidation Processes for Wastewater Treatment Using Accumulated Oxygen-Equivalent Criteria. Water Res. 2021, 200, 117234.
- Faria, P.C.C.; Monteiro, D.C.M.; Órfão, J.J.M.; Pereira, M.F.R. Cerium, Manganese and Cobalt Oxides as Catalysts for the Ozonation of Selected Organic Compounds. Chemosphere 2009, 74, 818–824.
- Nawrocki, J. Catalytic Ozonation in Water: Controversies and Questions. Discussion Paper. Appl. Catal. B 2013, 142–143, 465–471.
- Liotta, L.F.; Gruttadauria, M.; di Carlo, G.; Perrini, G.; Librando, V. Heterogeneous Catalytic Degradation of Phenolic Substrates: Catalysts Activity. J. Hazard. Mater. 2009, 162, 588–606.
- Nawrocki, J.; Fijołek, L. Catalytic Ozonation—Effect of Carbon Contaminants on the Process of Ozone Decomposition. Appl. Catal. B 2013, 142–143, 307–314.
- Wang, B.; Xiong, X.; Ren, H.; Huang, Z. Preparation of MgO Nanocrystals and Catalytic Mechanism on Phenol Ozonation. RSC Adv. 2017, 7, 43464–43473.
- Liu, X.; Zhu, W.; Yang, Z.; Yang, Y.; Li, H. Efficient Ozone Catalysis by Manganese Iron Oxides/Activated Carbon for Sulfamerazine Degradation. J. Water Process Eng. 2022, 49, 103050.
- Ikhlaq, A.; Brown, D.R.; Kasprzyk-Hordern, B. Mechanisms of Catalytic Ozonation on Alumina and Zeolites in Water: Formation of Hydroxyl Radicals. Appl. Catal. B 2012, 123–124, 94–106.
- Ikhlaq, A.; Waheed, S.; Joya, K.S. Catalytic Ozonation of Paracetamol on Zeolite A: Non-Radical Mechanism. Catal. Commun. 2018, 112, 15–20.
- Nawaz, F.; Cao, H.; Xie, Y.; Xiao, J.; Chen, Y.; Ghazi, Z.A. Selection of Active Phase of MnO2 for Catalytic Ozonation of 4-Nitrophenol. Chemosphere 2017, 168, 1457–1466.
- Nawaz, F.; Xie, Y.; Cao, H.; Xiao, J.; Wang, Y.; Zhang, X.; Li, M.; Duan, F. Catalytic Ozonation of 4-Nitrophenol over an Mesoporous α-MnO2 with Resistance to Leaching. Catal. Today 2015, 258, 595–601.
- Yu, G.; Wang, Y.; Cao, H.; Zhao, H.; Xie, Y. Reactive Oxygen Species and Catalytic Active Sites in Heterogeneous Catalytic Ozonation for Water Purification. Environ. Sci. Technol. 2020, 54, 5931–5946.
- Zada, A.; Khan, M.; Khan, M.A.; Khan, Q.; Habibi-Yangjeh, A.; Dang, A.; Maqbool, M. Review on the Hazardous Applications and Photodegradation Mechanisms of Chlorophenols over Different Photocatalysts. Environ. Res. 2021, 195, 110742.
- Gottschalk, C.; Libra, J.A.; Saupe, A. Ozonation of Water and Waste Water: A Practical Guide to Understanding Ozone and Its Applications, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2010.
- Rayaroth, M.P.; Aravindakumar, C.T.; Shah, N.S.; Boczkaj, G. Advanced Oxidation Processes (AOPs) Based Wastewater Treatment—Unexpected Nitration Side Reactions—A Serious Environmental Issue: A Review. Chem. Eng. J. 2022, 430, 133002.
- Inchaurrondo, N.S.; Font, J. Clay, Zeolite and Oxide Minerals: Natural Catalytic Materials for the Ozonation of Organic Pollutants. Molecules 2022, 27, 2151.
- Iqbal, J.; Shah, N.S.; Khan, Z.U.H.; Rizwan, M.; Murtaza, B.; Jamil, F.; Shah, A.; Ullah, A.; Nazzal, Y.; Howari, F.; et al. Visible Light Driven Doped CeO2 for the Treatment of Pharmaceuticals in Wastewater: A Review. J. Water Process Eng. 2022, 49, 103130.
- Hama Aziz, K.H. Application of Different Advanced Oxidation Processes for the Removal of Chloroacetic Acids Using a Planar Falling Film Reactor. Chemosphere 2019, 228, 377–383.
- Gardoni, D.; Vailati, A.; Canziani, R. Decay of Ozone in Water: A Review. Ozone Sci. Eng. 2012, 34, 233.
- Su, W.; Li, Y.; Hong, X.; Lin, K.Y.A.; Tong, S. Catalytic Ozonation of N, N-Dimethylacetamide in Aqueous Solution by Fe3O4@SiO2@MgO Composite: Optimization, Degradation Pathways and Mechanism. J. Taiwan Inst. Chem. Eng. 2022, 135, 104380.
- Weiner, F.R.; Matthews, A.R. Measurement of Water Quality. In Environmental Engineering; Butterworth Heinemann: Oxford, UK, 2003.
- Bai, Z.; Wang, J.; Yang, Q. Iron Doped Fibrous-Structured Silica Nanospheres as Efficient Catalyst for Catalytic Ozonation of Sulfamethazine. Environ. Sci. Pollut. Res. 2018, 25, 10090–10101.
- Jothinathan, L.; Hu, J. Kinetic Evaluation of Graphene Oxide Based Heterogenous Catalytic Ozonation for the Removal of Ibuprofen. Water Res. 2018, 134, 63–73.
- Faria, P.C.C.; Órfão, J.J.M.; Pereira, M.F.R. Ozonation of Aniline Promoted by Activated Carbon. Chemosphere 2007, 67, 809–815.
- Liu, Y.; Zhou, A.; Liu, Y.; Wang, J. Enhanced Degradation and Mineralization of 4-Chloro-3-Methyl Phenol by Zn-CNTs/O3 System. Chemosphere 2018, 191, 54–63.
- Maezono, T.; Tokumura, M.; Sekine, M.; Kawase, Y. Hydroxyl Radical Concentration Profile in Photo-Fenton Oxidation Process: Generation and Consumption of Hydroxyl Radicals during the Discoloration of Azo-Dye Orange II. Chemosphere 2011, 82, 1422–1430.
- Valdés, H.; Sánchez-Polo, M.; Rivera-Utrilla, J.; Zaror, C.A. Effect of Ozone Treatment on Surface Properties of Activated Carbon. Langmuir 2002, 18, 2111–2116.
- Morales-lara, F.; Pe, M.J.; Altmajer-vaz, D.; Garc, M.; Melguizo, M.; Lo, F.J. Functionalization of Multiwall Carbon Nanotubes by Ozone at Basic PH. Comparison with Oxygen Plasma and Ozone in Gas Phase. J. Phys. Chem. 2013, 117, 11647–11655.
- Razumovskii, S.D.; Gorshenev, V.N.; Kovarskii, A.L.; Kuznetsov, A.M.; Shchegolikhin, A.N. Carbon Nanostructure Reactivity: Reactions of Graphite Powders with Ozone. Fuller. Nanotub. Carbon Nanostruct. 2007, 15, 53–63.
- Álvarez, P.M.; García-Araya, J.F.; Beltrán, F.J.; Masa, F.J.; Medina, F. Ozonation of Activated Carbons: Effect on the Adsorption of Selected Phenolic Compounds from Aqueous Solutions. J. Colloid. Interface Sci. 2005, 283, 503–512.
- Psaltou, S.; Kaprara, E.; Triantafyllidis, K.; Mitrakas, M.; Zouboulis, A. Heterogeneous Catalytic Ozonation: The Significant Contribution of PZC Value and Wettability of the Catalysts. J. Environ. Chem. Eng. 2021, 9, 106173.
- Sánchez-Polo, M.; Rivera-Utrilla, J. Effect of the Ozone-Carbon Reaction on the Catalytic Activity of Activated Carbon during the Degradation of 1,3,6-Naphthalenetrisulphonic Acid with Ozone. Carbon 2003, 41, 303–307.
This entry is offline, you can click here to edit this entry!
