Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Plant Sciences
Most tree fruits are commercially grown on different root systems, hence called composite plants. The section provides the root system as the rootstock, and the atop ground portion is called the scion. The combination is selected based on different traits of scion varieties, rootstock, and prevailing edaphic situations. Rootstock selection is one of the most important factors in orchard management because it affects the growth, nutrient accumulation, environmental tolerance, and fruit quality of scion varieties.
- graft-union
- hormones
- molecular breeding
- rootstock
1. Introduction
Horticulture is currently a major potential sector for increasing agricultural production, income generation, and nutritional security through export, employment generation, value addition, and diversification. In addition to new major challenges, low productivity per unit area continues a problem in most of the horticultural crops, with climate change impacting the greater effect on fruit productivity. Biotic (causal agents of disease, insect, nematode, etc.), and abiotic (temperature, humidity, drought, wind, water logging, salinity, etc.), stresses are also challenges. Numerous studies on graft union formation and graft compatibility between scion–rootstock plants have given rise to several scientific hypotheses in herbaceous plants. However, due to long juvenile periods and generation times, and large plant size, studies at different growth stages of grafts in woody fruit plants are meager [1,2].
Rootstock selection is one of the most important factors in orchard management because it affects the growth, nutrient accumulation, environmental tolerance, and fruit quality of scion varieties [2,3,4]. Conventional breeding must be supplemented with molecular approaches to refine biotic and abiotic stress tolerance in fruit crops as lack of knowledge, breeding programs will often be time-consuming and costly. The identification of molecular markers linked to desirable rootstock traits must first be identified. For example, DNA fragments (genes), may be related to the production of an enzyme that promotes fruit set. If the location of this genetic material (marker) has been determined and the benefits of the marker have been identified, DNA from other rootstocks can be quickly screened for the gene of interest [5]. Parental genetic maps and delineated genomic regions associated with graft (in)-compatibility parameters in apricot has been studied by Pina et al. [6]. Therefore, the selection of desirable rootstocks at the nursery seedling stages can help to reduce the testing duration and exposure required for expensive field trials. Small interfering RNAs (silencing RNA) are now being used to grow virus-resistant transgenic rootstocks [7]. In rootstocks, the potential movement of RNA and DNA genetic or epigenetic factors and the transformation of proteins can be evaluated because of their unique and identifiable characteristics. It is commonly believed that grafted rootstocks and scions maintain their genetic identity, transcription factors, regulatory small interfering RNAs (siRNAs), micro RNAs (miRNAs), mRNAs, peptides, and proteins are mobile in the plant vascular system and may cross the graft union [8,9].
2. Molecular Mechanism between Scion and Rootstock
Earlier, researchers focused on the role of hormones in vascular reconnection [10]. Now, a successful graft union formation is the function of molecular signaling via phloem which leads to the anatomical and physiological changes in both components for the smooth connectivity of vascular tissues [11,12]. It is an established fact that the transfer of the intact plastid genome is essential across the graft junction at the molecular level for the uninterrupted communication between the scion and rootstock of the grafted plant [13,14]. The protein migrates from the companion cells of the shoot into the root cells and controls various physiological processes in plants [9]. The rapid development of molecular biotechnology and “omics” approaches will allow researchers to unravel the physiological and molecular mechanisms involved in the rootstock–scion interaction [15].
DNA, RNA, and Protein Transfer during Rootstock–Scion Interaction
As essential components of the RdDM pathway in Arabidopsis, sRNAs migrate bidirectional (from shoot to root and vice versa) [7]. The graft union allows molecules such as DNA, RNA, proteins, and hormones to be transferred bidirectionally between both the component of grafted plants [16,17]as shown in Figure 1.
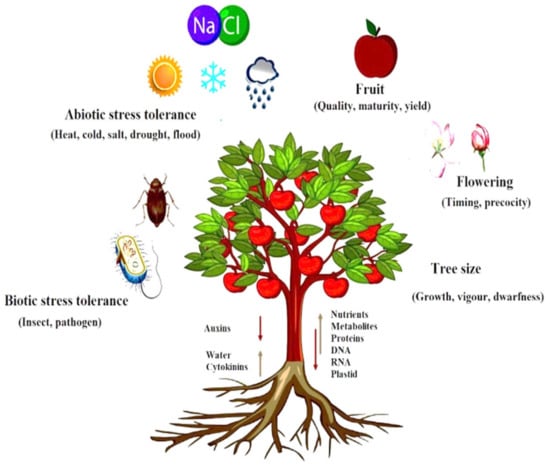
Figure 1. Macromolecules DNA, RNA, protein, hormones, and plastid DNA bidirectional movement via graft union explain the role of molecular and hormone signaling and also explain the effect of scion–rootstock relationship on quality fruit production.
DNA is able to transfer from rootstock to scion via vascular uptake. During the graft union formation, generation of plasmodesmata and re-establishment of vascular bundles provide horizontal gene transfer (HGT) transport channels [14,18,19]. Plant graft hybridization may result in the horizontal transfer of nuclear DNA and the transfer of cpDNA genetic material between the rootstock and scion, but no nuclear gene exchange occurs [19]. The entire chloroplast genome can be transferred through inter-specific grafting, regardless of graft orientation, and is relatively stable [20]. The movement of the nuclear genome occurs between grafting sections, resulting in the development of stable allopolyploid and fertile plants [21].
Small RNA regulates epigenetic alteration by counteracting DNA methylation in grafted and budded plants [22]. Small interfering RNAs (siRNAs) play an important role in signaling and gene silencing [12,23]. Micro RNAs (miRNAs) play a role during the development of graft union for example cca-miR159 and cca-miR156 are upregulated at the graft site of hickory plants during graft growth, stimulating the tissue attachment process. Furthermore, miR390b is downregulated, resulting in an increase in ATP-binding protein accumulation [24]. Superoxide radical scavenging enzyme superoxide dismutase 4 (SOD4) helps in the protection of plant cells due to oxidative stress caused during the early stages of the grafting due to tissue injury. SOD4 is a target of the miRNA cca-miR827, which has been found to be down-regulated during the early stages of grafting, preferring SOD4 activation to scavenge harmful superoxide radicals at graft unions [20]. Messenger RNAs (mRNAs), which are primarily responsible for the coding of various proteins, also regulate various functional proteins involved in the normal growth and development of plant tissues after they have been transported through the graft unions [25,26,27].
Many proteins (such as chaperones) have the ability to bind mRNAs and are known for their roles in promoting molecular transport and preventing mRNA degradation. One of these chaperones CmPP16 has been found in Cucurbita maxima (winter squash) and play role in RNA transfer from the rootstock to the scion via graft union [28]. The cyclophilin protein SICyp1 is transported from the scion to the rootstock via the phloem. It increases auxin response and helps to promote root development. Proteins from the companion cells of the shoot can be transferred into the stele cells of the roots and regulate the physiology of grafted plants [29,30,31]. Plasma membrane intrinsic proteins (PIPs) are involved in the grafting process, and aquaporins are involved in cellular water transport controlling active cell proliferation [32,33]. At the grafting site, stimulated expression of an aquaporin (PIP1B) is accompanied by cell elongation and increased water levels, leading to good callus formation [34,35].
The role of differentially expressed proteins (DEPs) in grafting had been observed. In hickory plants, 369 and 341 DEPs are found to be downregulated and upregulated at graft unions, respectively [36], and increased expression of CcPIP1;2 during grafting has been found to be functional in Carya cathayensis [37]. Guo et al. [38] studied the differentially expressed genes (DEGs) to understand the mechanism of pumpkin grafting's effect on watermelon fruit ripening and quality development. Loupit and Cookson [39] well explained the changes in transcript abundance during graft union formation indicate that grafting responses are similar to responses to wounding and include the differential expression of genes related to hormone signaling, oxidative stress, formation of new vascular vessels, cell development, and secondary metabolites, in particular polyphenols. The details of the differential expression of protein are shown in Table 1.
Table 1. Details of various proteins and their differential expression in horticultural crops.
| Protein | Response | Crop | Reference |
|---|---|---|---|
| CmPP16 | Favoring the process of molecular transport and reduce the degradation of mRNAs. | Cucurbita maxima | [28] |
| PbPTB3 | Play role in long-distance movement of mRNAs across the graft junction by binding of PbPTB3 to PbWoxT1 mRNA. |
Pyrus betlaefolia | [31] |
| Cyclophilin, SICyp1 | Play role in increased auxin response and promoting the growth of roots. | Tomato | [40] |
| PIP1B | Enhanced water levels and cell elongation, leading to better callus formation and successful grafting. | Carya cathayensis | [35] |
| DEPs | At graft unions, 341 and 369 DEPs were found to be upregulated. | Carya cathayensis | [41] |
| PIN | Reunion of vascular tissues is favored by the auxin movement from top to downward direction mediated by PIN proteins. | Arabidopsis | [42] |
This entry is adapted from the peer-reviewed paper 10.3390/agriculture12122036
This entry is offline, you can click here to edit this entry!
