Oxygenated polyunsaturated fatty acids (oxylipins) are bioactive molecules established as important mediators during inflammation. Different classes of oxylipins have been found to have opposite effects, e.g., pro-inflammatory prostaglandins and anti-inflammatory resolvins. Production of the different classes of oxylipins occurs during distinct stages of development and resolution of inflammation. Chronic inflammation is involved in the progression of many pathophysiological conditions and diseases such as non-alcoholic fatty liver disease, insulin resistance, diabetes, and obesity. Determining oxylipin profiles before, during, and after inflammatory-related diseases could provide clues to the onset, development, and prevention of detrimental conditions.
- eicosanoid
- inflammation
- diagnosis
- progression
- mass spectrometry
1. Introduction
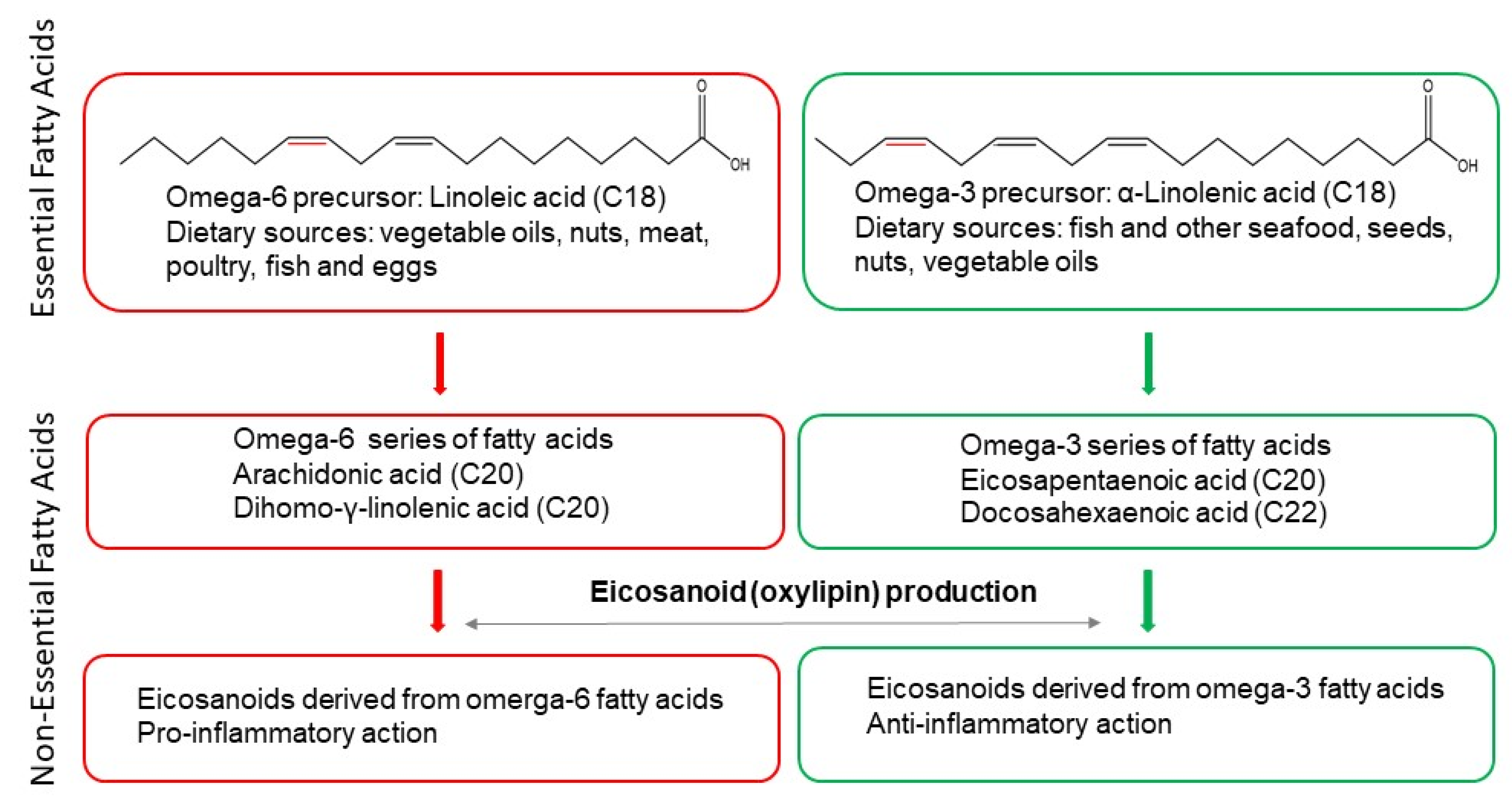
2. Non-Alcoholic Fatty Liver Disease (NAFLD)
3. Obesity and Diabetes
4. Technological Advancements
The importance of oxylipins in physiological and pathophysiological processes is underscored by their role in the ability of cells to acquire different functional phenotypes depending on the microenvironment. Omega-3 and omega-6 PUFA-derived oxylipins can modulate the inflammatory phenotype of cells [39] by acting as ligands for receptors such as the peroxisome proliferator-activated receptor PPAR [40] and prostaglandin E2 (PGE2) receptor PTGER4 [41].
The eicosanoid class comprises hundreds of structurally and stereochemically distinct species derived either enzymatically or non-enzymatically from a handful of precursors. It is therefore not surprising that within the group of eicosanoids can be found isomers (e.g., PGE2 and PGF2α) with different biological functions [14]. In addition to their diversity, eicosanoids occupy a small mass range and are found in low nanomolar concentrations in biological samples (e.g., human plasma and murine bone marrow-derived macrophages [42]). Thus, their detection and evaluation require methods that are sensitive, selective, and reproducible.
Mass spectrometry is a powerful technique for identifying and quantifying known and unknown analytes, and offers high specificity and selectivity. Technical advances have given mass spectrometry a range of tools to obtain information at the molecular level from samples, such as determining the molecular weight of an analyte, its separation from isomeric and isobaric species, its chemical formula, its molecular structure, and structural information [43][44][45]. Mass spectrometry can be used to investigate a wide range of classes of molecules, including those with small and large molecular weights, volatile and non-volatile, polar and non-polar [46][47][48].
Applications using mass spectrometry are either targeted or untargeted. Untargeted approaches attempt to measure as many compounds as possible in a sample, and can be used to discover unanticipated changes between experimental groups. This information can then be used to generate and test hypotheses, for example by applying targeted approaches which offer a selection of the best possible conditions for the detection, identification, and (absolute or relative) quantitation of particular analytes of choice, and is usually used in follow-up experiments. Targeted approaches may use multiple reaction monitoring (MRM) methods which use the knowledge of the mass of the ionised analyte and its corresponding fragments formed during the mass spectrometry analysis [49][50].
For lipidomics, liquid chromatography-mass spectrometry (LC-MS) is commonly used. LC separates molecules depending on their hydrophobicity, molecular size, and polarity, and covers a broad range of non-polar and weakly polar analytes [51][52]. Another separation method that is gaining popularity in metabolomics is ion mobility coupled with mass spectrometry (IMS-MS; IM-MS) (IMS) [42][53][54][55][56][57][58].
Recently, supercritical fluid chromatography (SFC) has gained traction as an alternative technique to LC in lipidomics due to its high efficiency [59][60]. Briefly, SFC uses supercritical fluid such as CO2 as a mobile phase [61], which results in high separation efficiency and short separation time [62][63]. SFC-MS/MS methods have been reported to detect inflammation-related lipids including oxylipins in rats [64], as well as for the simultaneous measurement of five AA-derived metabolites (PGD2, PGE2, PGF2α, 6KetoPGF1α and LTB4) in biological samples [65]. Thus, SFC coupled to MS is a promising approach for the interrogation of the lipidome.
Novel ionisation approaches have appeared with the development of ambient mass spectrometry, characterised by direct sampling and ionisation of the analytes with no or minimal sample preparation [66]. Ambient ionisation MS has been successfully employed to rapidly differentiate bacterial species based on their lipid profiles [67]. Mass spectrometry imaging (MSI) developments allow more detailed investigations of biological questions such as the biochemical origin of lipid spatial distribution [68]. Examples of MSI techniques include secondary ion mass spectrometry (SIMS), Desorption electrospray ionisation (DESI) and matrix-assisted desorption/ionisation (MALDI). Lipid characterisation is now possible due to technological advances in SIMS [69]. DESI-MSI can record 2D distributions of polar lipids in tissue slices at ambient conditions (at atmospheric pressure) [70], and has been developed for the simultaneous imaging of polar and non-polar lipids in mouse brain tissue [71]. Improvements in MALDI resolution resulted in the identification and localisation of lipids within the kidney, as well as the localisation of lipid droplets with lesion-specific macrophages [72]. Thus, further method developments could provide the means to image eicosanoids in a variety of biological samples.
This entry is adapted from the peer-reviewed paper 10.3390/metabo12121238
References
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875.
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361.
- Simmons, D.L.; Botting, R.M.; Hla, T. Cyclooxygenase isozymes: The biology of prostaglandin synthesis and inhibition. Pharm. Rev 2004, 56, 387–437.
- Smith, W.L.; DeWitt, D.L.; Garavito, R.M. Cyclooxygenases: Structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000, 69, 145–182.
- Capdevila, J.H.; Falck, J.R.; Imig, J.D. Roles of the cytochrome P450 arachidonic acid monooxygenases in the control of systemic blood pressure and experimental hypertension. Kidney Int. 2007, 72, 683–689.
- Fleming, I. DiscrEET regulators of homeostasis: Epoxyeicosatrienoic acids, cytochrome P450 epoxygenases and vascular inflammation. Trends Pharm. Sci 2007, 28, 448–452.
- Imig, J.D. Targeting epoxides for organ damage in hypertension. J. Cardiovasc. Pharm. 2010, 56, 329–335.
- Roman, R.J. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol. Rev. 2002, 82, 131–185.
- Spector, A.A.; Norris, A.W. Action of epoxyeicosatrienoic acids on cellular function. Am. J. Physiol. Cell Physiol. 2007, 292, C996–C1012.
- Williams, J.M.; Murphy, S.; Burke, M.; Roman, R.J. 20-hydroxyeicosatetraeonic acid: A new target for the treatment of hypertension. J. Cardiovasc. Pharm. 2010, 56, 336–344.
- Balić, A.; Vlašić, D.; Žužul, K.; Marinović, B.; Bukvić Mokos, Z. Omega-3 Versus Omega-6 Polyunsaturated Fatty Acids in the Prevention and Treatment of Inflammatory Skin Diseases. Int. J. Mol. Sci. 2020, 21, 741.
- Schmitz, G.; Ecker, J. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 2008, 47, 147–155.
- Muhlhausler, B.S.; Cook-Johnson, R.; James, M.; Miljkovic, D.; Duthoit, E.; Gibson, R. Opposing effects of omega-3 and omega-6 long chain polyunsaturated Fatty acids on the expression of lipogenic genes in omental and retroperitoneal adipose depots in the rat. J. Nutr. Metab. 2010, 2010, 927836.
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523.
- Yamaguchi, A.; Botta, E.; Holinstat, M. Eicosanoids in inflammation in the blood and the vessel. Front. Pharm. 2022, 13, 997403.
- Sheppe, A.E.F.; Edelmann, M.J. Roles of Eicosanoids in Regulating Inflammation and Neutrophil Migration as an Innate Host Response to Bacterial Infections. Infect. Immun. 2021, 89, e0009521.
- Mendoza, S.R.; Zamith-Miranda, D.; Takacs, T.; Gacser, A.; Nosanchuk, J.D.; Guimaraes, A.J. Complex and Controversial Roles of Eicosanoids in Fungal Pathogenesis. J. Fungi 2021, 7, 254.
- Artru, F.; McPhail, M.J.W.; Triantafyllou, E.; Trovato, F.M. Lipids in Liver Failure Syndromes: A Focus on Eicosanoids, Specialized Pro-Resolving Lipid Mediators and Lysophospholipids. Front. Immunol. 2022, 13, 867261.
- Bedossa, P. Pathology of non-alcoholic fatty liver disease. Liver Int. 2017, 37, 85–89.
- Teli, M.R.; James, O.F.; Burt, A.D.; Bennett, M.K.; Day, C.P. The natural history of nonalcoholic fatty liver: A follow-up study. Hepatology 1995, 22, 1714–1719.
- Zoller, H.; Tilg, H. Nonalcoholic fatty liver disease and hepatocellular carcinoma. Metabolism 2016, 65, 1151–1160.
- Cotter, T.G.; Rinella, M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology 2020, 158, 1851–1864.
- Golabi, P.; Paik, J.; Hwang, J.P.; Wang, S.; Lee, H.M.; Younossi, Z.M. Prevalence and outcomes of non-alcoholic fatty liver disease (NAFLD) among Asian American adults in the United States. Liver Int. 2019, 39, 748–757.
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84.
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218.
- Banaszczak, M.; Maciejewska, D.; Drozd, A.; Ryterska, K.; Milc, D.J.; Raszeja-Wyszomirska, J.; Wunsch, E.; Gonzalez-Muniesa, P.; Stachowska, E. 5-Lipooxygenase Derivatives as Serum Biomarkers of a Successful Dietary Intervention in Patients with NonAlcoholic Fatty Liver Disease. Medicina 2020, 56, 58.
- Kalveram, L.; Schunck, W.H.; Rothe, M.; Rudolph, B.; Loddenkemper, C.; Holzhutter, H.G.; Henning, S.; Bufler, P.; Schulz, M.; Meierhofer, D.; et al. Regulation of the cytochrome P450 epoxyeicosanoid pathway is associated with distinct histologic features in pediatric non-alcoholic fatty liver disease. Prostaglandins Leukot. Essent. Fat. Acids 2021, 164, 102229.
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The Diagnosis and Management of Non-alcoholic Fatty Liver Disease: Practice Guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012, 142, 1592–1609.
- Dyson, J.K.; Anstee, Q.M.; McPherson, S. Non-alcoholic fatty liver disease: A practical approach to diagnosis and staging. Frontline Gastroenterol. 2014, 5, 211–218.
- Di Mauro, S.; Scamporrino, A.; Filippello, A.; Di Pino, A.; Scicali, R.; Malaguarnera, R.; Purrello, F.; Piro, S. Clinical and Molecular Biomarkers for Diagnosis and Staging of NAFLD. Int. J. Mol. Sci. 2021, 22, 11905.
- Tuomisto, K.; Palmu, J.; Long, T.; Watrous, J.D.; Mercader, K.; Lagerborg, K.A.; Andres, A.; Salmi, M.; Jalkanen, S.; Vasan, R.S.; et al. A plasma metabolite score of three eicosanoids predicts incident type 2 diabetes: A prospective study in three independent cohorts. BMJ Open Diabetes Res. Care 2022, 10, e002519.
- Miao, Z.J.; Tang, X.; Schultzberg, M.; Zhao, Y.W.; Wang, X.Z. Plasma Resolvin D2 to Leukotriene B-4 Ratio Is Reduced in Diabetic Patients with Ischemic Stroke and Related to Prognosis. Biomed. Res. Int. 2021, 2021, 6657646.
- Tans, R.; Bande, R.; van Rooij, A.; Molloy, B.J.; Stienstra, R.; Tack, C.J.; Wevers, R.A.; Wessels, H.; Gloerich, J.; van Gool, A.J. Evaluation of cyclooxygenase oxylipins as potential biomarker for obesity-associated adipose tissue inflammation and type 2 diabetes using targeted multiple reaction monitoring mass spectrometry. Prostaglandins Leukot. Essent. Fat. Acids 2020, 160, 102157.
- Pawelzik, S.C.; Avignon, A.; Idborg, H.; Boegner, C.; Stanke-Labesque, F.; Jakobsson, P.J.; Sultan, A.; Back, M. Urinary prostaglandin D2 and E2 metabolites associate with abdominal obesity, glucose metabolism, and triglycerides in obese subjects. Prostaglandins Other Lipid Mediat. 2019, 145, 106361.
- Truchan, N.A.; Fenske, R.J.; Sandhu, H.K.; Weeks, A.M.; Patibandla, C.; Wancewicz, B.; Pabich, S.; Reuter, A.; Harrington, J.M.; Brill, A.L.; et al. Human Islet Expression Levels of Prostaglandin E2 Synthetic Enzymes, But Not Prostaglandin EP3 Receptor, Are Positively Correlated with Markers of beta-Cell Function and Mass in Nondiabetic Obesity. ACS Pharm. Transl. Sci. 2021, 4, 1338–1348.
- Fisk, H.L.; Childs, C.E.; Miles, E.A.; Ayres, R.; Noakes, P.S.; Paras-Chavez, C.; Kuda, O.; Kopecky, J.; Antoun, E.; Lillycrop, K.A.; et al. Modification of subcutaneous white adipose tissue inflammation by omega-3 fatty acids is limited in human obesity-a double blind, randomised clinical trial. EBioMedicine 2022, 77, 103909.
- Sanchez-Fernandez, A.; Zandee, S.; Mastrogiovanni, M.; Charabati, M.; Rubbo, H.; Prat, A.; Lopez-Vales, R. Administration of Maresin-1 ameliorates the physiopathology of experimental autoimmune encephalomyelitis. J. Neuroinflammation 2022, 19, 27.
- Dieckmann, S.; Maurer, S.; Fromme, T.; Colson, C.; Virtanen, K.A.; Amri, E.Z.; Klingenspor, M. Fatty Acid Metabolite Profiling Reveals Oxylipins as Markers of Brown but Not Brite Adipose Tissue. Front. Endocrinol. 2020, 11, 73.
- Chistyakov, D.V.; Gavrish, G.E.; Goriainov, S.V.; Chistyakov, V.V.; Astakhova, A.A.; Azbukina, N.V.; Sergeeva, M.G. Oxylipin Profiles as Functional Characteristics of Acute Inflammatory Responses in Astrocytes Pre-Treated with IL-4, IL-10, or LPS. Int. J. Mol. Sci. 2020, 21, 780.
- Chistyakov, D.V.; Astakhova, A.A.; Goriainov, S.V.; Sergeeva, M.G. Comparison of PPAR Ligands as Modulators of Resolution of Inflammation, via Their Influence on Cytokines and Oxylipins Release in Astrocytes. Int. J. Mol. Sci. 2020, 21, 9577.
- Na, Y.R.; Jung, D.; Stakenborg, M.; Jang, H.; Gu, G.J.; Jeong, M.R.; Suh, S.Y.; Kim, H.J.; Kwon, Y.H.; Sung, T.S.; et al. Prostaglandin E2 receptor PTGER4-expressing macrophages promote intestinal epithelial barrier regeneration upon inflammation. Gut 2021, 70, 2249–2260.
- Hinz, C.; Liggi, S.; Mocciaro, G.; Jung, S.; Induruwa, I.; Pereira, M.; Bryant, C.E.; Meckelmann, S.W.; O′Donnell, V.B.; Farndale, R.W.; et al. A Comprehensive UHPLC Ion Mobility Quadrupole Time-of-Flight Method for Profiling and Quantification of Eicosanoids, Other Oxylipins, and Fatty Acids. Anal. Chem. 2019, 91, 8025–8035.
- Mabud, M.D.A.; Dekrey, M.J.; Cooks, R.G. Surface-Induced Dissociation of Molecular-Ions. Int. J. Mass Spectrom. 1985, 67, 285–294.
- Syka, J.E.; Coon, J.J.; Schroeder, M.J.; Shabanowitz, J.; Hunt, D.F. Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc. Natl. Acad. Sci. USA 2004, 101, 9528–9533.
- Zubarev, R.A.; Horn, D.M.; Fridriksson, E.K.; Kelleher, N.L.; Kruger, N.A.; Lewis, M.A.; Carpenter, B.K.; McLafferty, F.W. Electron capture dissociation for structural characterization of multiply charged protein cations. Anal. Chem. 2000, 72, 563–573.
- Chait, B.T. Mass spectrometry in the postgenomic era. Annu. Rev. Biochem. 2011, 80, 239–246.
- Kind, T.; Fiehn, O. Advances in structure elucidation of small molecules using mass spectrometry. Bioanal. Rev. 2010, 2, 23–60.
- McCullagh JSO, O.N. Mass Spectrometry; Oxford University Press: Oxford, UK, 2019.
- Mann, M.; Hendrickson, R.C.; Pandey, A. Analysis of proteins and proteomes by mass spectrometry. Annu. Rev. Biochem. 2001, 70, 437–473.
- Chen, G.Y.; Zhang, Q.B. Comprehensive analysis of oxylipins in human plasma using reversed-phase liquid chromatography-triple quadrupole mass spectrometry with heatmap-assisted selection of transitions. Anal. Bioanal. Chem. 2019, 411, 367–385.
- Theodoridis, G.A.; Gika, H.G.; Want, E.J.; Wilson, I.D. Liquid chromatography-mass spectrometry based global metabolite profiling: A review. Anal. Chim. Acta 2012, 711, 7–16.
- Molnar, I.; Horvath, C. Reverse-phase chromatography of polar biological substances: Separation of catechol compounds by high-performance liquid chromatography. Clin. Chem. 1976, 22, 1497–1502.
- Kanu, A.B.; Dwivedi, P.; Tam, M.; Matz, L.; Hill, H.H., Jr. Ion mobility-mass spectrometry. J. Mass Spectrom. 2008, 43, 1–22.
- Lanucara, F.; Holman, S.W.; Gray, C.J.; Eyers, C.E. The power of ion mobility-mass spectrometry for structural characterization and the study of conformational dynamics. Nat. Chem. 2014, 6, 281–294.
- Hill, H.H., Jr.; Siems, W.F.; St Louis, R.H.; McMinn, D.G. Ion mobility spectrometry. Anal. Chem. 1990, 62, 1201A–1209A.
- Dodds, J.N.; Baker, E.S. Ion Mobility Spectrometry: Fundamental Concepts, Instrumentation, Applications, and the Road Ahead. J. Am. Soc. Mass Spectrom. 2019, 30, 2185–2195.
- Paglia, G.; Smith, A.J.; Astarita, G. Ion mobility mass spectrometry in the omics era: Challenges and opportunities for metabolomics and lipidomics. Mass Spectrom. Rev. 2022, 41, 722–765.
- Harris, R.A.; Leaptrot, K.L.; May, J.C.; McLean, J.A. New Frontiers in Lipidomics Analyses using Structurally Selective Ion Mobility-Mass Spectrometry. Trends Anal. Chem. 2019, 116, 316–323.
- Chollet, C.; Boutet-Mercey, S.; Laboureur, L.; Rincon, C.; Mejean, M.; Jouhet, J.; Fenaille, F.; Colsch, B.; Touboul, D. Supercritical fluid chromatography coupled to mass spectrometry for lipidomics. J. Mass Spectrom. 2019, 54, 791–801.
- Yang, Y.; Liang, Y.; Yang, J.; Ye, F.; Zhou, T.; Gongke, L. Advances of supercritical fluid chromatography in lipid profiling. J. Pharm. Anal. 2019, 9, 1–8.
- Taylor, L.T. Supercritical fluid chromatography. Anal. Chem. 2010, 82, 4925–4935.
- Saito, M. History of supercritical fluid chromatography: Instrumental development. J. Biosci. Bioeng. 2013, 115, 590–599.
- Kalikova, K.; Slechtova, T.; Vozka, J.; Tesarova, E. Supercritical fluid chromatography as a tool for enantioselective separation; A review. Anal. Chim. Acta 2014, 821, 1–33.
- Jin, W.; Yang, J.; Liu, D.; Zhong, Q.; Zhou, T. Determination of inflammation-related lipids in depressive rats by on-line supercritical fluid extraction-supercritical fluid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2021, 203, 114210.
- Kumari, A.U.S.J.; Acharya, S.R.; Bergquist, J. A novel, fast and sensitive supercritical fluid chromatography-tandem mass spectrometry (SFC-MS/MS) method for analysis of arachidonic acid metabolites. Analyst 2018, 143, 3661–3669.
- Qi, K.; Wu, L.; Liu, C.; Pan, Y. Recent Advances of Ambient Mass Spectrometry Imaging and Its Applications in Lipid and Metabolite Analysis. Metabolites 2021, 11, 780.
- Su, H.; Jiang, Z.H.; Chiou, S.F.; Shiea, J.; Wu, D.C.; Tseng, S.P.; Jain, S.H.; Chang, C.Y.; Lu, P.L. Rapid Characterization of Bacterial Lipids with Ambient Ionization Mass Spectrometry for Species Differentiation. Molecules 2022, 27, 2772.
- Bowman, A.P.; Heeren, R.M.A.; Ellis, S.R. Advances in mass spectrometry imaging enabling observation of localised lipid biochemistry within tissues. TrAC Trends Anal. Chem. 2019, 120, 115197.
- Passarelli, M.K.; Winograd, N. Lipid imaging with time-of-flight secondary ion mass spectrometry (ToF-SIMS). Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2011, 1811, 976–990.
- Takats, Z.; Wiseman, J.M.; Gologan, B.; Cooks, R.G. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science 2004, 306, 471–473.
- Liu, C.; Qi, F.; Pan, Y. Imaging of Polar and Nonpolar Lipids Using Desorption Electrospray Ionization/Post-photoionization Mass Spectrometry. Methods Mol. Biol. 2021, 2306, 285–298.
- Bowman, A.P.; Bogie, J.F.J.; Hendriks, J.J.A.; Haidar, M.; Belov, M.; Heeren, R.M.A.; Ellis, S.R. Evaluation of lipid coverage and high spatial resolution MALDI-imaging capabilities of oversampling combined with laser post-ionisation. Anal. Bioanal. Chem. 2020, 412, 2277–2289.
