In cardiorenal syndrome (CRS), heart failure and renal failure are pathophysiologically closely intertwined by the reciprocal relationship between cardiac and renal injury. Type 1 CRS is most common and associated with acute heart failure. A preexistent chronic kidney disease (CKD) is common and contributes to acute kidney injury (AKI) in CRS type 1 patients (acute cardiorenal syndrome). The remaining CRS types are found in patients with chronic heart failure (type 2), acute and chronic kidney diseases (types 3 and 4), and systemic diseases that affect both the heart and the kidney (type 5). Establishing the diagnosis of CRS requires various tools based on the type of CRS, including non-invasive imaging modalities such as TTE, CT, and MRI, adjuvant volume measurement techniques, invasive hemodynamic monitoring, and biomarkers.
1. Introduction
The network organ interactions of the heart and kidneys are intimately involved in the pathophysiology of cardiorenal syndrome (CRS) [
1,
2]. In addition, interactions of the heart and kidneys with the central nervous system (CNS) contribute to CRS [
3]. The first bibliographic reference to this relationship was made almost 200 years ago, in 1836, by Robert Bright, who observed structural changes in the heart in patients with chronic kidney disease [
4]. Although the functional relationship between the heart and the kidneys had long been anticipated, the Working Group of the National Heart, Lung, and Blood Institute tried for the first time to define the syndrome in 2004 [
5]. The Working Group stated, “In heart failure, it is the result of interactions between the kidneys and other circulatory compartments that increase circulating volume and symptoms of heart failure and disease progression are exacerbated. At its extreme, cardio-renal dysregulation leads to what is termed “cardio-renal syndrome” in which therapy to relieve congestive symptoms of heart failure is limited by further decline in renal function.” In 2008, the Acute Dialysis Quality Initiative introduced a less cardio-centric definition, dividing the forms of CRS into two categories, cardiorenal and renocardiac syndromes [
1]. Currently, five types of the syndrome are classified [
6], as summarized in
Table 1.
Table 1. Classification and basic characteristics of cardiorenal syndrome (CRS). AHF: acute heart failure; AKI: acute kidney injury; ACS: acute coronary syndrome; CHF: chronic heart failure; CKD: chronic kidney disease.
Various organ systems and mechanisms contribute to the pathophysiology of the syndrome, including hemodynamic changes, neurohormonal mechanisms, inflammatory reactions, oxidative stress mechanisms, and various less-defined mechanisms. Various biomarkers assessing heart function and damage, glomerular filtration, and renal tubular damage are currently used or under study to diagnose and assess the prognosis of CRS. Further diagnostic opportunities offered by cardiac ultrasound and magnetic resonance imaging (MRI) can be used to evaluate CRS patients [
7]. Various endovascular volume assessment techniques are available today, which can provide important insights into the course of the disease and contribute to clinical decision-making. The pillars of the treatment strategy of the syndrome include decongestion therapy, the use of vasodilators and inotropic agents, and inhibition of RAAS. In addition to clinical signs and symptoms of heart failure, CRS may also contribute to supraventricular arrhythmias, especially atrial fibrillation and sudden cardiac death [
8]. CRS has also recently been associated with impaired prognosis in COVID-19 disease [
9]. Newer drugs and therapies, such as SGLT2 inhibitors, tolvaptan, and cardiac resynchronization therapy (CRT), have now been suggested as potential therapeutic agents for CRS.
2. CRS Types and Epidemiological Data
CRS is a group of disorders that bear out the reciprocal relationship between cardiac and renal injury. Different observational and retrospective studies have shown the prevalence and burden of each of the five types of CRS. CRS type 1 is the most common. The nature of epidemiologic data limits clear delineation between cardiorenal syndrome types 2 and 4. Overall, the presence of cardiac or renal dysfunction strongly inhabits a poor prognosis of the contrary organ [
10].
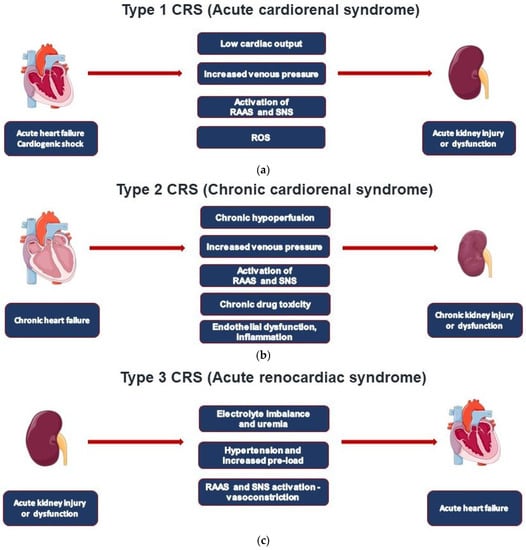
Type 1 CRS (acute cardiorenal) is characterized by the acute worsening of cardiac function leading to AKI (
Figure 1a) [
11]. This type occurs in about 25% of hospitalized cases with acute decompensated heart failure (ADHF) [
12]. A preexistent chronic kidney disease (CKD) is common and linked to acute kidney injury (AKI) in 60% of all patients studied. AKI can be considered an independent mortality risk factor in ADHF patients, including those with ST myocardial infarction and/or reduced left ventricular ejection fraction (LVEF) [
11].
Figure 1. Pathogenic pathways involved in the five CRS types.
CRS type 2 is characterized by chronic pathological changes in cardiac function leading to kidney injury or dysfunction, and chronic renal disease has been observed in 45-63% of chronic heart failure (CHF) patients (
Figure 1b). However, it may be difficult to classify these patients, which often include those shifting from a clinical condition of type 1 CRS [
11]. Of note, the initial decline of glomerular filtration rate (GFR) regularly observed after initiation of heart failure medications (ACEi, ARB, ARNI, SGLT2i) results from the reduction in glomerular pressure, but the decline in GFR either slows or remains unchanged from natural course [
13,
14].
Type 3 CRS is characterized by acute worsening of kidney function leading to heart disease. A wide spectrum of cardiac dysfunction includes cases with acute decompensated heart failure, acute coronary syndrome, and arrhythmias as defined by the RIFLE (Risk, Injury, Failure, Loss, End-stage kidney disease) and AKIN (Acute Kidney Injury Network) criteria (
Figure 1c) [
11,
15]. AKI actually represents an independent cardiovascular risk factor for mortality in hospitalized patients, especially in those on renal replacement therapy (RRT).
AKI seems to involve almost 70% of patients in ICUs, where 5–25% of patients can develop severe AKI, with mortality rates ranging from 50 to 80% [
16]. ADHF still represents the most common acute cardiac dysfunction syndrome worldwide, and it can be defined as new-onset or gradual or rapid worsening of preexistent heart failure with signs and symptoms requiring immediate therapy [
17]. Cardiac valvular disease, atrial fibrillation, arterial hypertension, as well as noncardiac comorbidities (renal dysfunction, diabetes, anemia) and medications (especially non-steroidal anti-inflammatory drugs and glitazones) can contribute to ADHF development [
17,
18]. Renal dysfunction affects mortality rates in ADHF patients from 1.9% (mild renal disease) to 7.6% (severe renal dysfunction) [
17].
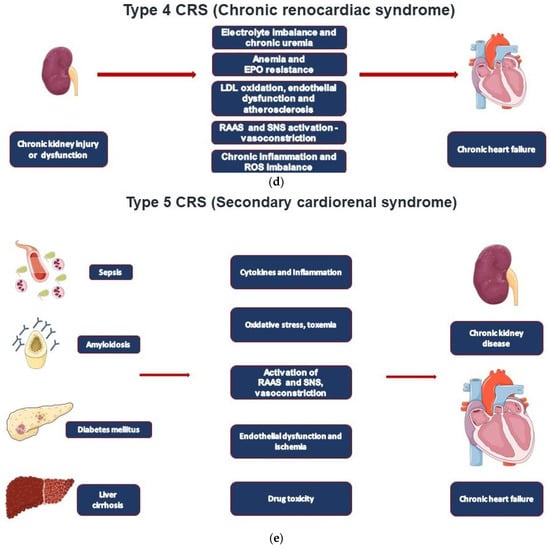
Type 4 CRS, also defined as chronic renocardiac disease, is characterized by cardiovascular involvement in patients affected by CKD at any stage (
Figure 1d). It is well established that renal dysfunction is an independent risk factor for cardiovascular disease with a higher mortality risk for myocardial infarction and sudden death in CKD. A meta-analysis by Tonelli et al. conducted on 1.4 million patients found higher mortality rates for all causes with eGFR decline with relative death odds ratios of 1.9, 2.6, and 4.4 for GFR levels of 80, 60, and 40 mL/min, respectively [
19]. The largest epidemiological study was actually performed by Go et al. on over 1 million people; cardiovascular risk was found particularly evident in patients with stages IIIb-IV (according to the K/DOQI CKD classification) renal disease and in those who underwent RRT (hemodialysis, peritoneal dialysis, and transplantation) [
20]. The Chronic Renal Insufficiency Cohort (CRIC) Study focused on 190 patients presenting stage III to end-stage renal disease and performing serial echocardiographic exams; in the 2-year evaluation period in which patients shifted from stage V to end-stage renal disease, the EF dropped from 53 to 50%; therefore, they found that the number of subjects with EF <50% increased by 20% [
21].
Type 5 CRS takes place when cardiac and renal injury occur at the same time as it occurs in sepsis and in systemic inflammatory response syndrome (SIRS) (
Figure 1e) [
18]. Type 5 CRS is involved in COVID-19-associated CRS [
9]. Type 5 CRS is a recently defined clinical syndrome; thus, solid epidemiological data are not available.
This entry is adapted from the peer-reviewed paper 10.3390/jcm11237041