The nucleotide excision repair (NER) pathway is the most universal repair pathway for the removal of a wide range of structurally unrelated DNA lesions, including UV photolesions (e.g., cyclobutane pyrimidine dimers (CPDs) and pyrimidine-pyrimidone (6-4)-photoproducts (6-4PPs)), intrastrand crosslinks, reactive oxygen species-induced base alterations, and bulky adducts of DNA bases with reactive metabolites of some chemical carcinogens or chemotherapeutic agents [
1,
2,
3]. Mutations in NER-related genes are associated with an autosomal recessive disease called xeroderma pigmentosum (XP) [
4]. XP is characterized by extreme sensitivity of the skin to sunlight and a dramatically increased risk of skin cancer [
5,
6]. A subset of XP patients developed a profound neurodegenerative condition known as XP neurological disease [
7]. XP patients can be classified into seven complementation groups, XP-A through XP-G, depending on the specific gene that is affected [
1]. Patients with known mutations in the
XPA gene have the most severe form of XP, indicating a critical role of the XPA protein in the NER process.
2. XPA’s Structure and DNA-Binding and Protein-Binding Abilities
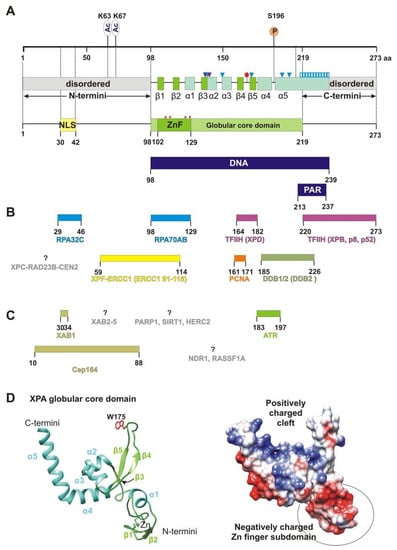
XPA is one of the smallest proteins inside the NER machine: human XPA is 31 kDa and consists of only 273 amino acid residues (aa). XPA is composed of a central globular domain (aa 98–219) that is flanked by dynamically disordered N and C termini (
Figure 1A). This kind of structural organization, when structured globular domains are combined with disordered regions, is very common among eukaryotic proteins [
23]. Frequently, disordered proteins (entirely disordered or containing disordered sequences, as in XPA) interact with or function as hubs in protein interaction networks or play a central role in an ordered assembly of macromolecular machines [
24,
25]. Indeed, both low-sequence-complexity parts enable the XPA protein to interact with a variety of protein partners. Future experiments will shed light on disordered XPA part properties: do they do folding upon binding to protein partners (and should be called “intrinsically disordered”), or do these regions not adopt a specific three-dimensional structure during functioning. In addition, the N terminus contains a conserved nuclear localization signal (NLS) that lies in the 13-residue stretch from aa 30 to 42 [
26,
27] (
Figure 1A). The NLS is a tag that ensures that the protein is sorted into the nucleus, but in the case of XPA, things are not so simple, and we discuss this matter below.
Figure 1. XPA’s structure and interaction partners. (A) The map of the XPA domain structure and known points of PTMs: phosphorylation at S196 and acetylation at K63 and K67. Secondary-structure elements are shown according to crystal structures PDB 6LAE and 6J44: β-strands are green (β1: aa 103–104, β2: aa 111–112, β3: aa 138–140, β4: aa 164–167, and β5: aa 178–172), and α-helices are light blue (α1: aa 116–121, α2: aa 141–148, α3: aa 151–157, α4: aa 183–194, and α5: aa 197–239). Positively charged residues K141, K151, K179, R207, and R211, which are directly involved in interactions with backbones of a DNA duplex, are shown as blue triangles. Two residues (Thr140 and Thr142, indicated as purple triangles) interact with the DNA backbone through a van der Waals contact and a hydrogen bond, respectively. Extended helix α5 contains several positively charged residues (Lys217/218/221/222/224/236 and Arg227/228/231/237) that are possibly involved in DNA binding, which are shown as a blue striped box. Conserved residue Trp175 intercalates into unpaired bases of single-stranded DNA (ssDNA) at the ss–dsDNA junction and is displayed as a red circle. Unstructured N- and C-terminal regions are gray. Zinc-coordinated conserved cysteine residues (C105, C108, C126, and C129) are presented as red asterisks and a Zn-finger motif (ZnF, aa 102–129) colored green. The N terminus accommodates a nuclear localization signal (NLS, aa 30–42), which is yellow. DNA-binding (aa 98–239) and poly(ADP-ribose) (PAR)-binding (aa 213–237) motifs are mapped to the overall XPA structure and are highlighted in dark blue. (B) Interaction sites for NER protein partners on XPA, which are aligned with the XPA residues involved in each interaction. Proteins whose interaction sites are unknown are gray. (C) XPA interaction partners outside NER. (D) A structural model of the XPA globular core domain (PDB ID: 6LAE). A ribbon diagram with color codes according to (A). The Trp175 residue is shown in red. A distribution of the electrostatic potential on the surface for the same structure: a positive charge is shown in blue, and a negative charge is red. The structures were generated using UCSF Chimera software (version 1.16).
The central domain contains a C4-type Zn-finger (ZnF) motif that has the sequence Cys
105-X
2-Cys
108-X
17-Cys
126-X
2-Cys
129 [
28] (
Figure 1A). Side chains of cysteines Cys105/108 and Cys126/129 coordinate the zinc ion [
14]. Although the ZnF of XPA is essential for DNA binding and for NER activity [
29], the ZnF core itself is not directly in contact with DNA [
16] but rather properly ensures the DBD folding [
30]. Moreover, the zinc-containing subdomain is even negatively charged due to many glutamate and aspartate residues [
14] (
Figure 1D). Notably, the UvrA protein has two units of the same C4 type of the ZnF motif [
28].
Originally, the minimal DNA-binding domain (DBD) of XPA was mapped to a central globular core between residues 98 and 219 [
14,
15,
29,
31,
32]. This region contains a sheet-helix-hairpin motif (residues 138–182) and a helix-turn-helix motif (residues 183–230) that form a shallow clamp-like or right hand-like structure (the sheet-helix-hairpin motif as fingers and helix-turn-helix as a thumb) with a positively charged inner surface (
Figure 1D). The internal curvature of the basic cleft fits well to the diameter of a standard B-form dsDNA [
14]. Subsequent studies have found that some residues on the C-terminal side beyond the minimal DBD domain are also involved in binding to DNA substrates [
33,
34,
35]. Later, the XPA DBD was redefined and extended by 20 additional C-terminal residues (Asp220–Thr239). The redefined XPA DBD (aa 98–239) can bind to DNA with an affinity nearly identical to that of the full-length XPA protein [
34,
35]. The crystal structure revealed that the C-terminal extension folds as a long α-helix (α5 in
Figure 1A) with a basic residue cluster resulting in the formation of a consecutive positively charged surface [
16,
17]. Interestingly, structural superposition of the human XPA DBD on a yeast Rad14–DNA complex implies that the α5 extension (Asp217–Thr239) cannot directly come into contact with a DNA substrate.
XPA has an ability to recognize some bulky lesions [
22,
36] and especially prefers to bind to kinked and branched DNA structures [
37,
38,
39]. All of these DNA structures contain an ss–dsDNA junction. XPA binds to the duplex part of the junction in a non-sequence-specific manner via electrostatic interactions between the positively charged cleft and negatively charged phosphate backbones of the DNA duplex [
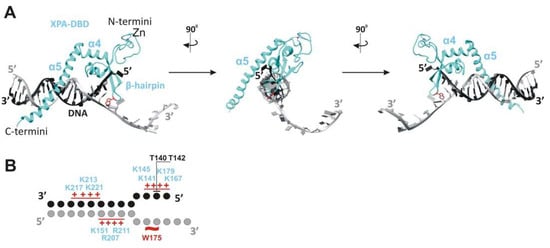
17] (
Figure 1F and
Figure 2). This intermediate is further stabilized by hydrogen bonding of the side chain hydroxyl group of Thr142, and a van der Waals contact is formed between the side chain Cβ atom of Thr140 and DNA’s phosphate moieties (
Figure 1A and
Figure 2). Thereafter, Trp175 from the top of the hairpin between β4 and β5 is stacked with bases of the unpaired ssDNA at the junction, thereby giving rise to a stable conformation of this β-hairpin [
17,
21]. According to the energy calculations, binding to ssDNA in the 3′→5′ direction is more favorable than that in the 5′→3′ direction (relative to α5), but it is not observed experimentally [
29,
38].
Figure 2. XPA interaction with the ss–dsDNA junction. (
A) Cryo-EM structure PDB: 6RO4 provides details of the XPA–DBD interaction with the ss–dsDNA junction. XPA demarcates the 5′ edge of the DNA repair bubble. XPA inserts its intercalating β-hairpin between DNA single strands at the junction. Red colored Trp175 from the tip of the β-hairpin stacks against the base of the DNA 3′-extension at the junction. The structures were generated using UCSF Chimera software (version 1.16). (
B) Schematic representation of the interactions between side chains of the XPA and DNA junction, according to cryo-EM structure PDB: 6RO4 [
21] and crystal structure PDB: 6LAE. DNA nucleotides are indicated as circles. Patches of positively charged residues in proximity to the DNA backbone are indicated by red pluses. Hydrogen bonding of T142 and a van der Waals contact of T140 are indicated as black lines.
Recently, it was shown with atomic force microscopy, scanning force microscopy, and mathematical modeling that XPA undergoes episodic one-dimensional diffusion to search the DNA for damage [
40]. The functional meaning of XPA’s damage recognition ability is not clear, and today, XPA is considered only as a protein scaffold element inside the NER complex. Anyway, XPA interacts with the proteins involved in every step of NER, from damage recognition to DNA synthesis. Yet, it is unknown how many contacts XPA could engage in concurrently, and it is possible that XPA interacts with these proteins not simultaneously but in the order of them joining the repair machinery.
Figure 1B lists XPA’s protein interaction partners directly involved in NER according to the following process steps:
2.1. Initial Damage Recognition
XPA interacts physically with DDB2 through aa 185–226, and this interaction can be seen both in vitro and in vivo [
41]. UV-damaged DNA-binding protein (DDB1/2) is a heterodimeric protein consisting of subunits DDB1 and DDB2/XPE and has an extraordinarily high binding affinity and specificity for CPD and 6-4PP [
2,
3]. The biological role of the XPA–DDB2 interaction is unclear, but because the DDB2 interaction site overlaps with the poly(ADP-ribose) [PAR]-binding motif (aa 213–237), we can speculate that the XPA–DDB2 complex is involved in PAR-dependent chromatin remodeling together with PARP1 and XPC [
42]. XPC (which functions in the complex with proteins RAD23B and CEN2) is the protein sensor responsible for the detection of a wide variety of DNA lesions that are repaired through the global genome NER (GG-NER) pathway [
43]. XPA interacts with XPC and stimulates its binding to the DNA [
44,
45]. The XPC interaction site in the XPA sequence and the biological meaning of this interaction are unknown.
2.2. Damage Verification
The TFIIH complex is the key protein for the damage verification step. Depending on the context, the TFIIH composition changes from a core of seven subunits, including translocase XPB and helicase XPD, to ten subunits, through the addition of three CAK (Cdk-activating kinase module) kinase subunits [
21,
46]. XPA’s whole C terminus is involved in an interaction with the TFIIH protein [
47]. Recent cryo-EM data expanded our knowledge about the TFIIH–XPA interaction [
21]. XPA (together with nuclease XPG) facilitates the CAK kinase module release from the core TFIIH and stabilizes an alternative conformation of TFIIH, where the XPD helicase assumes the open conformation for the functioning. In this complex, XPA forms a bridge between the XPB and the XPD, and moreover, XPA’s extended α5 helix and ATPase XPB form a positively charged tunnel that holds the DNA within. Thus, by trapping the DNA within a duplex tunnel, XPA may keep the NER machinery on the DNA during lesion scanning and processing [
21].
2.3. Pre-Incision Complex Formation
Immediately after TFIIH opens the DNA repair bubble, the undamaged ssDNA that is being formed is bound by replication protein A (RPA) [
48]. RPA is a heterotrimer consisting of subunits RPA70, RPA32, and RPA14 [
49,
50]. XPA interacts with two of them: RPA70 (which contains OB-fold domains A, B, and C) and RPA32 (which contains the D OB-fold domain) [
51]. It was shown recently that the interaction between XPA (aa 29–46) and RPA32C is important for the initial association of XPA with NER complexes, whereas the interaction between XPA (aa 98–126) and RPA70AB is needed for structural shaping of the complex to enable the dual incision reaction [
52]. Pre-incision complex formation is completed by the engagement of nuclease XPF–ERCC1, which is recruited by the XPA through the interaction with ERCC1 [
53,
54,
55,
56,
57]. XPA aa 59–114 are responsible for this interaction. In particular, the Gly72–Phe75 region (also known as the G motif) and the Glu78–Glu84 region (i.e., the E motif) are the residues necessary for the binding of XPF–ERCC1. Biochemical data have shown that the ZnF motif (aa 102–129) is partially involved in this contact, but the NMR and molecular dynamics simulation revealed that only the 14-amino acid sequence (aa 67–80) mediates this interaction.
2.4. Dual Incision, Resynthesis, and Ligation
After the first incision, proliferating cell nuclear antigen (PCNA) joins the NER complex. PCNA is the processivity factor for DNA polymerases. XPA has been found to interact directly with PCNA via the APIM sequence (the AlkB homolog 2 PCNA-interacting motif), and it has been shown that XPA and PCNA colocalize to the damaged DNA foci in a cell culture [
58,
59,
60]. XPA
−/− cells complemented with XPA containing a mutated APIM sequence have high UV sensitivity and a deficient repair of CPDs and 6-4PPs and are consequently more arrested in the S phase as compared to XPA
−/− cells complemented with wild-type XPA. Notably, XPA colocalizes with PCNA in the replication foci and is loaded onto a newly synthesized DNA in undamaged cells; thus, it is possible that this interaction is required for DNA-processing pathways other than NER.
2.5. XPA Dimerization
It is well-known that isolated XPA easily forms a homodimer. Moreover, in vitro, it can form the XPA
2–RPA complex [
61]. Instead, XPA has been widely assumed to be a monomer participating in the mechanism of NER (the first statement about the monomeric functional form was published by [
62]). Accordingly, the physiological meaning of the XPA dimerization and the structural mechanism of this process are still unclear. Recently obtained molecular dynamics simulation data indicate that some residues make a contribution to the intermolecular interactions in XPA homodimers, but this needs to be validated by another approach [
63]. Notably, XPA is not the sole NER protein that demonstrates easily dimerization characters, but as in the case of XPA, there is no functional explanation found for this ability [
64].
2.6. PTM Proteins
Today, it is known that XPA is precisely tuned by several PTMs [
13,
65,
66]. Obviously, XPA interacts with proteins that provide these modifications and removes them, but we know the interaction site only for the ATR kinase: the α4 helix [
67] (
Figure 1C). Interaction sites with proteins facilitating these modifications—RASSF1A and NDR1—have not been determined either. The only interaction site that has been identified is the one for the Cep164 protein, which functions in ATR-mediated checkpoint activation; it interacts with XPA through aa 10–88 of XPA [
68].
2.7. XABs
Using the yeast two-hybrid system, researchers identified a novel set of XPA-interacting proteins that was designated as XABs [
69]. XAB1 is a cytoplasmic protein with GTPase activity and binds to the N-terminal region (residues 1–52) of XPA, and the region “aa 30–34” is directly involved in this interaction (
Figure 1C). The XAB1-interacting site overlaps with the NLS and raises a question about XAB1′s role in the cytoplasmic sequestering of XPA. Among the five found XABs, only XAB2 has a nuclear function and is intensively investigated. It has been reported that XAB2 interacts with the proteins involved in transcription-coupled NER (TC-NER), for example, CSA, CSB, and RNA polymerase II [
70]. XAB2 contains 15 TPR (tetratricopeptide repeat) motifs and appears to have a role in transcription and pre-mRNA splicing [
71]. Generally, XAB2 serves as a guardian of POLR2A (the largest catalytic subunit of RNA polymerase II) expression to ensure global gene expression and to antagonize cell senescence [
72]. Furthermore, XAB2 promotes homologous recombination and facilitates histone acetylation events linked to homologous recombination [
73]. Immunoprecipitation studies have revealed that XAB2 interacts with endonucleases ERCC1–XPF and XPG outside NER under the conditions that favor the formation of R-loops [
74]. Altogether, XAB2 is involved in R-loop removal and pre-mRNA splicing; both processes are linked to transcription [
75]. Future experiments will shed light on the possible involvement of XPA in processes where XAB2 is active.