Contributors : Francesca Gaeta Francesco De Caro Gianluigi Franci Pasquale Pagliano Pietro Vajro Claudia Mandato
Monkeypox disease has been endemic in sub-Saharan Africa for a long time, attracting remarkable attention only in 2022 through the occurrence of a multi-country outbreak. The latter has raised serious public health concerns and is considered a public health emergency by the World Health Organization. Although the disease is usually self-limiting, it can cause severe illness in individuals with compromised immune systems, in children, and/or the pregnant woman–fetus dyad. Patients generally present with fever, lymphadenopathy, and a vesicular rash suggestive of mild smallpox. Serious eye, lung and brain complications, and sepsis can occur.
- antivirals
- breastfeeding
- children
- monkeypox
- newborn
- outbreak
- pregnancy
- prevention
- testing
- vaccination
1. Introduction
2. Monkeypox Virus: Generalities
3. Clinical Presentation, Transmission and Prevention
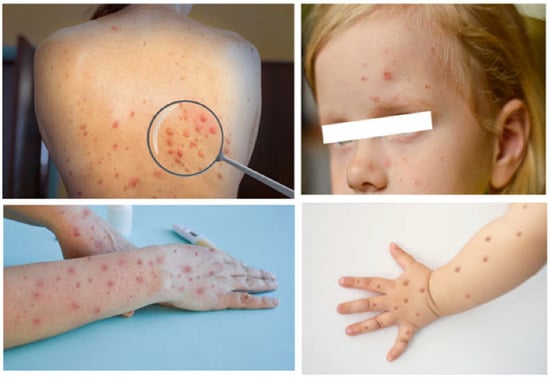
4. Diagnosis
5. Treatment
5.1. Antiviral Medicines
- (a)
-
Cidofovir (Vistide) is primarily used as a treatment for retinitis, encephalitis and oesophagitis caused by cytomegalovirus, especially in people with HIV. It is the phosphorylated active metabolite of brincidovir. In-vitro and preclinical studies showed that is effective against poxviruses [4];
- (b)
-
Brincidofovir (Tembexa) is available as oral suspension/tablets, approved byFood and Drug Administration (FDA) for smallpox disease [49]. Both drugs are inhibitors of DNA replication with a broad spectrum of activity against multiple families of double-stranded DNA viruses.
- (c)
-
Tecovirimat (ST-246): is an antiviral medication which impairs the function of the VP37 envelope protein necessary for the formation of the extracellular enveloped virus required for cell-to-cell transmission; it has more specific activity against orthopoxviruses [49]. It has been approved by FDA, used to treat human smallpox disease but can be used against MPV. Tecovirimat is given orally (TPOXX®: 200 mg capsule) or as an injectable formulation [41]. Capsules should be taken within 30 min after a full meal with moderate to high fat. Per CDC guidelines, for those who cannot swallow they can be opened and mixed with liquids/soft food [50] Because it is an inducer of cytochrome P450 (CYP) 3A and CYP2B6, co-administration with this drug may lead to reduced plasma exposures of sensitive substrates of CYP3A4 or CYP2B6, reducing the effects. Because of the presence in its IV formulation of a potentially nephrotoxic substance (hydroxypropyl-β-cyclodextrin), it is advisable to dose creatinine clearance (CrCl) and liver function before starting treatment. Intravenous therapy is safe in mild/moderate renal impairment but is contraindicated in severe nephropathies (CrCl < 30 mL/min), both in adults and children [43]. Dose adjustments for oral therapy instead are not required in the case of mild, moderate, severe nephropathy or even in patients requiring hemodialysis in end-stage renal disease [51]. Although reduced fertility due to testicular toxicity was found in mouse models, no human data are available [51].
5.2. Children’s Treatment
Further detailed and up-to-date information on:
- antiviral drugs treatment during pregnancy and breast-feeding
- vaccines for pre- and post-exposure prophylaxis at all ages
- population, ethics and risk of discrimination
is available inside the article by Gaeta et al. Monkeypox Infection 2022: An Updated Narrative Review Focusing on the Neonatal and Pediatric Population. Children 2022, 9, 1832. https://doi.org/10.3390/children9121832
6. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/children9121832
References
- Moore, M.J.; Rathish, B.; Zahra, F. Monkeypox; Stat Pearls Publishing LLC: Treasure Island, FL, USA, 2022. Available online: www.ncbi.nlm.nih.gov/books/NBK574519/ (accessed on 18 November 2022).
- Ladnyj, I.D.; Ziegler, P.; Kima, E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World Health Organ. 1972, 46, 593–597.
- Reynolds, M.G.; Yorita, K.L.; Kuehnert, M.J.; Davidson, W.B.; Huhn, G.D.; Holman, R.C.; Damon, I.K. Clinical Manifestations of Human Monkeypox Influenced by Route of Infection. J. Infect. Dis. 2006, 194, 773–780.
- Kumar, N.; Acharya, A.; Gendelman, H.E.; Byrareddy, S.N. The 2022 outbreak and the pathobiology of the monkeypox virus. J. Autoimmun. 2022, 131, 102855.
- CDC. 2022 Monkeypox Outbreak Global Map. Available online: https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html (accessed on 18 November 2022).
- ECDC. 2022 Monkeypox Situation Update. Available online: https://www.ecdc.europa.eu/en/news-events/monkeypox-situation-update (accessed on 18 November 2022).
- WHO. Monkeypox Outbreak 2022. Available online: https://www.who.int/emergencies/situation-reports (accessed on 18 November 2022).
- Hennessee, I.; Shelus, V.; McArdle, C.E.; Wolf, M.; Schatzman, S.; Carpenter, A.; Minhaj, F.S.; Petras, J.K.; Cash-Goldwasser, S.; Maloney, M.; et al. Epidemiologic and Clinical Features of Children and Adolescents Aged < 18 Years with Monkeypox—United States, 17 May–24 September 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1407–1411.
- WHO Director-General Declares the Ongoing Monkeypox Outbreak a Public Health Emergency of International Concern. Available online: https://www.who.int/europe/news/item/23-07-2022-who-director-general-declares-the-ongoing-monkeypox-outbreak-a-public-health-event-of-international-concern (accessed on 18 November 2022).
- Alakunle, E.; Moens, U.; Nchinda, G.; Okeke, M. Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Viruses 2020, 12, 1257.
- Likos, A.M.; Sammons, S.A.; Olson, V.A.; Frace, A.M.; Li, Y.; Olsen-Rasmussen, M.; Davidson, W.; Galloway, R.; Khristova, M.L.; Reynolds, M.G.; et al. A tale of two clades: Monkeypox viruses. J. Gen. Virol. 2005, 86 Pt 10, 2661–2672.
- WHO. Variants Names. Available online: https://www.who.int/news/item/12-08-2022-monkeypox--experts-give-virus-variants-new-names (accessed on 18 November 2022).
- Agrati, C.; Cossarizza, A.; Mazzotta, V.; Grassi, G.; Casetti, R.; De Biasi, S.; Pinnetti, C.; Gili, S.; Mondi, A.; Cristofanelli, F.; et al. Immunological Signature in Human Cases of Monkeypox Infection in 2022 Outbreak. Lancet Infect. Dis. 2022.
- Johnston, S.C.; Johnson, J.C.; Stonier, S.W.; Lin, K.L.; Kisalu, N.K.; Hensley, L.E.; Rimoin, A.W. Cytokine modulation correlates with severity of monkeypox disease in humans. J. Clin. Virol. 2015, 63, 42–45.
- Sanjuán, R.; Domingo-Calap, P. Mechanisms of viral mutation. Cell. Mol. Life Sci. 2016, 73, 4433–4448.
- Gigante, C.M.; Plumb, M.; Ruprecht, A.; Zhao, H.; Wicker, V.; Wilkins, K.; Matheny, A. Genomic deletions and rearrangements in monkeypox virus from the 2022 outbreak, USA. bioRxiv 2022.
- Wassenaar, T.M.; Wanchai, V.; Ussery, D.W. Comparison of Monkeypox virus genomes from the 2017 Nigeria outbreak and the 2022 outbreak. J. Appl. Microbiol. 2022, 133, 3690–3698.
- Kozlov, M. The monkeypox virus is mutating. Are scientists worried? Nature 2022. epub ahead of print.
- Angelo, K.M.; Petersen, B.W.; Hamer, D.H.; Schwartz, E.; Brunette, G. Monkeypox transmission among international travellers—Serious monkey business? J. Travel Med. 2019, 26, taz002.
- York, A. The bodily distribution of monkeypox virus. Nat. Rev. Genet. 2022, 20, 703.
- Palich, R.; Burrel, S.; Monsel, G.; Nouchi, A.; Bleibtreu, A.; Seang, S.; Bérot, V.; Brin, C.; Gavaud, C.; Wakim, Y.; et al. Viral loads in clinical samples of men with monkeypox virus infection: A French case series. Lancet Infect. Dis. 2022.
- Lum, F.-M.; Torres-Ruesta, A.; Tay, M.Z.; Lin, R.T.P.; Lye, D.C.; Rénia, L.; Ng, L.F.P. Monkeypox: Disease epidemiology, host immunity and clinical interventions. Nat. Rev. Immunol. 2022, 22, 597–613.
- Di Gennaro, F.; Veronese, N.; Marotta, C.; Shin, J.I.; Koyanagi, A.; Silenzi, A.; Antunes, M.; Saracino, A.; Bavaro, D.F.; Soysal, P.; et al. Human Monkeypox: A Comprehensive Narrative Review and Analysis of the Public Health Implications. Microorganisms 2022, 10, 1633.
- Vouga, M.; Nielsen-Saines, K.; Dashraath, P.; Baud, D. The monkeypox outbreak: Risks to children and pregnant women. Lancet Child Adolesc. Health 2022, 6, 751–753.
- D’Antonio, F.; Pagani, G.; Buca, D.; Khalil, A. Monkeypox infection in pregnancy: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. MFM 2022, 5, 100747.
- CDC. Monkeypox in Animals. Available online: https://www.cdc.gov/poxvirus/monkeypox/veterinarian/monkeypox-in-animals.html (accessed on 18 November 2022).
- Grant, R.; Nguyen, L.-B.L.; Breban, R. Modelling human-to-human transmission of monkeypox. Bull. World Health Organ. 2020, 98, 638–640.
- WHO. Disease Outbreak News. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON396 (accessed on 18 November 2022).
- Pastula, D.M.; Tyler, K.L. An Overview of Monkeypox Virus and Its Neuroinvasive Potential. Ann. Neurol. 2022, 92, 527–531.
- CDC. Clinical Recognition. Available online: https://www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html (accessed on 18 November 2022).
- Sejvar, J.J.; Chowdary, Y.; Schomogyi, M.; Stevens, J.; Patel, J.; Karem, K.; Fischer, M.; Kuehnert, M.J.; Zaki, S.R.; Paddock, C.D.; et al. Human Monkeypox Infection: A Family Cluster in the Midwestern United States. J. Infect. Dis. 2004, 190, 1833–1840.
- Kaler, J.; Hussain, A.; Flores, G.; Kheiri, S.; Desrosiers, D. Monkeypox: A Comprehensive Review of Transmission, Pathogenesis, and Manifestation. Cureus 2022, 14, e26531.
- Adalja, A.; Inglesby, T. A Novel International Monkeypox Outbreak. Ann. Intern. Med. 2022, 175, 1175–1176.
- Nörz, D.; Pfefferle, S.; Brehm, T.T.; Franke, G.; Grewe, I.; Knobling, B.; Aepfelbacher, M.; Huber, S.; Klupp, E.M.; Jordan, S.; et al. Evidence of surface contamination in hospital rooms occupied by patients infected with monkeypox, Germany, June 2022. Eurosurveillance 2022, 27, 2200477.
- CDC. Isolation and Infection Control at Home. Available online: https://www.cdc.gov/poxvirus/monkeypox/clinicians/infection-control-home.html (accessed on 18 November 2022).
- Rash Illness Testing Protocol. Acute, Generalized Vesicular or Pustular Rash Illness Testing Protocol in the United States. Available online: https://www.cdc.gov/smallpox/lab-personnel/laboratory-procedures/rash-testing.html (accessed on 15 October 2022).
- Mccollum, A.M.; Damon, I.K. Human Monkeypox. Clin. Infect. Dis. 2014, 58, 260–267.
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; et al. Monkeypox Virus Infection in Humans across 16 Countries—April–June 2022. N. Engl. J. Med. 2022, 387, 679–691.
- Ramnarayan, P.; Mitting, R.; Whittaker, E.; Marcolin, M.; O’Regan, C.; Sinha, R.; Bennett, A.; Moustafa, M.; Tickner, N.; Gilchrist, M.; et al. Neonatal Monkeypox Virus Infection. N. Engl. J. Med. 2022, 387, 1618–1620.
- WHO. Laboratory Testing for the Monkeypox Virus: Interim Guidance 23 May 2022. Available online: https://www.who.int/publications/i/item/WHO-MPX-laboratory-2022.1 (accessed on 18 November 2022).
- Singhal, T.; Kabra, S.K.; Lodha, R. Monkeypox: A Review. Indian J. Pediatr. 2022, 89, 955–960.
- MacNeil, A.; Reynolds, M.; Braden, Z.; Carroll, D.S.; Bostik, V.; Karem, K.; Smith, S.K.; Davidson, W.; Li, Y.; Moundeli, A.; et al. Transmission of Atypical Varicella-Zoster Virus Infections Involving Palm and Sole Manifestations in an Area with Monkeypox Endemicity. Clin. Infect. Dis. 2009, 48, e6–e8.
- Monkeypox—AAP Book American Academy of Pediatrics. Available online: https://www.aap.org/en/patient-care/monkeypox/ (accessed on 18 November 2022).
- Dashraath, P.; Nielsen-Saines, K.; Mattar, C.; Musso, D.; Tambyah, P.; Baud, D. Guidelines for pregnant individuals with monkeypox virus exposure. Lancet 2022, 400, 21–22.
- Baud, D.; Nielsen-Saines, K.; Dashraath, P. Approach to monkeypox in pregnancy: Conjecture is best guided by evidence. Am. J. Obstet. Gynecol. 2022. epub ahead of print.
- Webb, E.; Rigby, I.; Michelen, M.; Dagens, A.; Cheng, V.; Rojek, A.M.; Dahmash, D.; Khader, S.; Gedela, K.; Norton, A.; et al. Availability, scope and quality of monkeypox clinical management guidelines globally: A systematic review. BMJ Glob. Health 2022, 7, e009838.
- CDC. Monkeypox. Available online: https://www.cdc.gov/poxvirus/monkeypox/if-sick/treatment.html#:~:text=There%20are%20no%20treatments%20specifically,and%20treat%20monkeypox%20virus%20infections (accessed on 18 November 2022).
- Adler, H.; Gould, S.; Hine, P.; Snell, L.B.; Wong, W.; Houlihan, C.F.; Osborne, J.C.; Rampling, T.; Beadsworth, M.B.; Duncan, C.J.; et al. NHS England High Consequence Infectious Diseases (Airborne) Network. Clinical features and management of human monkeypox: A retrospective observational study in the UK. Lancet Infect. Dis. 2022, 22, 1153–1162, Erratum in Lancet Infect. Dis. 2022, 22, e177.
- Siegrist, A.E.; Sassine, J. Antivirals with Activity Against Monkeypox: A Clinically Oriented Review. Clin. Infect. Dis. 2022. epub ahead of print.
- CDC. Guidance for Tecovirimat Use. Available online: https://www.cdc.gov/poxvirus/monkeypox/pdf/tecovirimat-ind-protocol-cdc-irb.pdf (accessed on 18 November 2022).
- Expanded Access IND Protocol: Use of Tecovirimat (TPOXX®) for Treatment of Human Non-Variola Orthopoxvirus Infections in Adults and Children IND No. 116,039 CDC IRB No. 6402 Version 6.1 10 August 2022. Available online: https://solanocounty.com/documents/TecovirimatINDProtocol_CDCIRB6402_v5.105.20.22_versionfor2022MPXOutbreak.pdf (accessed on 18 November 2022).
- O’Laughlin, K.; Tobolowsky, F.A.; Elmor, R.; Overton, R.; O’Connor, S.M.; Damon, I.K.; Petersen, B.W.; Rao, A.K.; Chatham-Stephens, K.; Yu, P.; et al. Clinical Use of Tecovirimat (Tpoxx) for Treatment of Monkeypox Under an Investigational New Drug Protocol—United States, May–August 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1190–1195.
- Rabaan, A.A.; Abas, A.H.; Tallei, T.E.; Al-Zaher, M.A.; Al-Sheef, N.M.; Fatimawali; Al-Nass, E.Z.; Al-Ebrahim, E.A.; Effendi, Y.; Idroes, R.; et al. Monkeypox Outbreak 2022: What We Know So Far and Its Potential Drug Targets and Management Strategies. J. Med. Virol. 2022. epub ahead of print.
- Clinical Considerations for Monkeypox in Children and Adolescents. Available online: https://www.cdc.gov/poxvirus/monkeypox/clinicians/pediatric.html (accessed on 18 November 2022).
- Red Book Online Outbreak: Monkeypox Virus Outbreak. Available online: https://publications.aap.org/redbook/resources/20705?autologincheck=redirected?nfToken=00000000-0000-0000-0000-000000000000 (accessed on 18 November 2022).
- Medscape Drug and Disease. Tecovirimat. Available online: https://reference.medscape.com/drug/tpoxx-tecovirimat-1000237 (accessed on 18 November 2022).
- EMA European Medicine Agency—Tecovirimat. Available online: www.ema.europa.eu/en/medicines/human/EPAR/tecovirimat-siga (accessed on 18 November 2022).
- Grosenbach, D.W.; Jordan, R.; Hruby, D.E. Development of the small-molecule antiviral ST-246® as a smallpox therapeutic. Futur. Virol. 2011, 6, 653–671.
- Rizk, J.G.; Lippi, G.; Henry, B.M.; Forthal, D.N.; Rizk, Y. Prevention and Treatment of Monkeypox. Drugs 2022, 82, 957–963.
- Forni, D.; Cagliani, R.; Molteni, C.; Clerici, M.; Sironi, M. Monkeypox virus: The changing facets of a zoonotic pathogen. Infect. Genet. Evol. 2022, 105, 105372.
- Mukherjee, A.G.; Wanjari, U.R.; Kannampuzha, S.; Das, S.; Murali, R.; Namachivayam, A.; Renu, K.; Ramanathan, G.; Doss, C.; Vellingiri, B.; et al. The pathophysiological and immunological background of the monkeypox virus infection: An update. J. Med. Virol. 2022. epub ahead of print.
- Technical Report 3. Available online: https://www.cdc.gov/poxvirus/monkeypox/cases-data/technical-report/report-3.html (accessed on 18 November 2022).
- Yao, K. The diversity of clinical manifestations of human monkeypox should be emphasized in practice. Pediatr. Investig. 2022, 6, 224–225.
