Catheter Ablation (CA) is an effective therapeutic option in treating atrial fibrillation (AF). Persistent AF represents the advanced stage during the progression of AF. “AF begets AF” is recognized as a main mechanism for the persistence of AF: a complex situation involving triggers and substrate (i.e., structural, electrical, and autonomic remodeling). Previous meta-analysis of RCTs including 809 persistent AF patients (mean age 60 years, mean LAD 46 mm) has already shown that PVI based CA is superior to AADs in preventing recurrence of atrial tachyarrhythmia among patients with persistent AF.
- atrial fibrillation
- ablation
- rhythm control
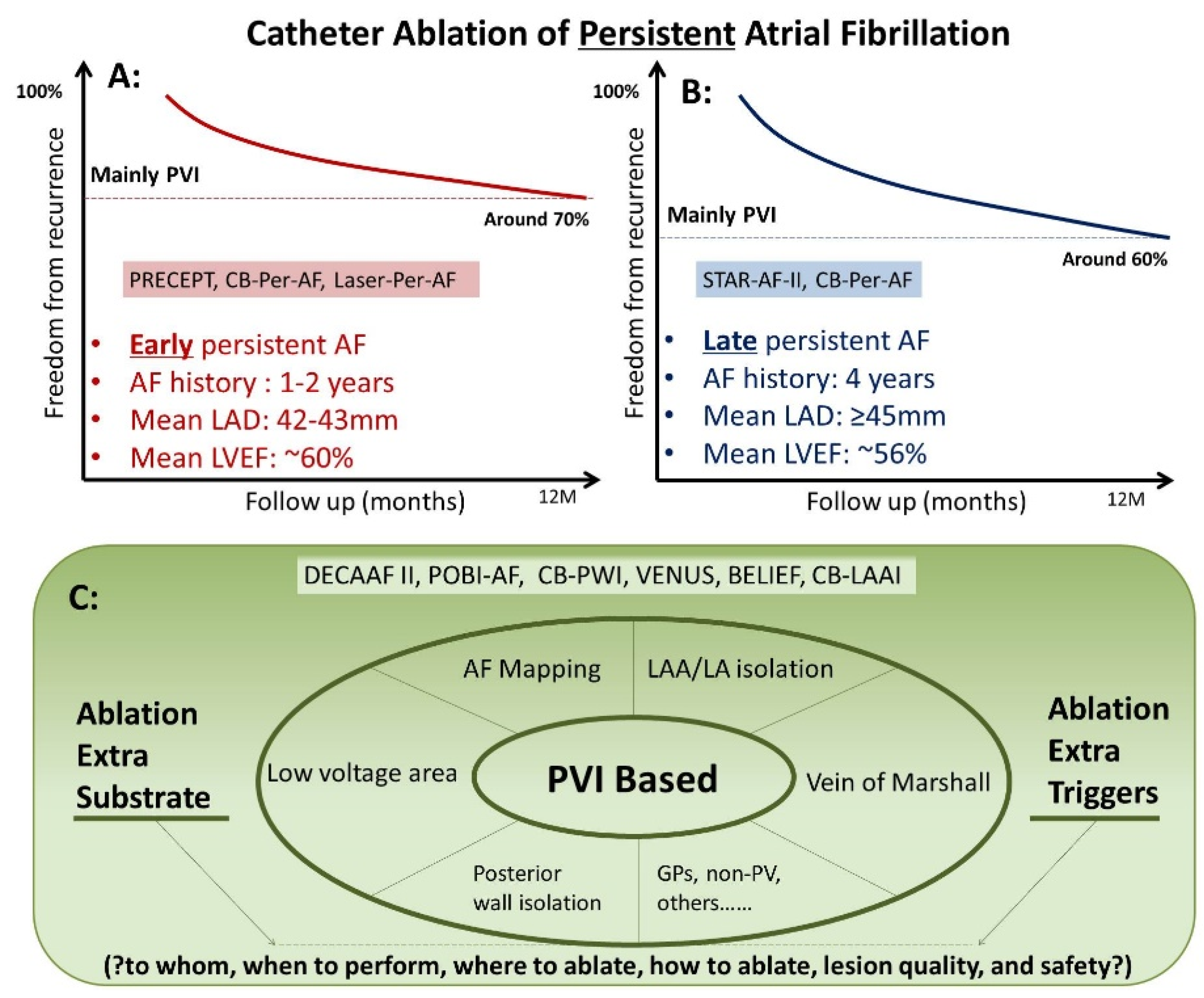
1. CA in Treating Persistent AF and Longstanding Persistent AF
2. Adjunctive Ablation Strategies beyond PVI in Treating Persistent AF
2.1. Ablation of Atrial Low Voltage Area
2.2. Left Atrial Posterior Wall Isolation
2.3. Ablation Vein of Marshall
2.4. Left Atrial Appendage Isolation/Ablation
This entry is adapted from the peer-reviewed paper 10.3390/jcm11226871
References
- Mansour, M.; Calkins, H.; Osorio, J.; Pollak, S.J.; Melby, D.; Marchlinski, F.E.; Athill, C.A.; Delaughter, C.; Patel, A.M.; Gentlesk, P.J.; et al. Persistent Atrial Fibrillation Ablation With Contact Force-Sensing Catheter: The Prospective Multicenter PRECEPT Trial. JACC Clin. Electrophysiol. 2020, 6, 958–969.
- Schmidt, B.; Neuzil, P.; Luik, A.; Osca Asensi, J.; Schrickel, J.W.; Deneke, T.; Bordignon, S.; Petru, J.; Merkel, M.; Sediva, L.; et al. Laser Balloon or Wide-Area Circumferential Irrigated Radiofrequency Ablation for Persistent Atrial Fibrillation: A Multicenter Prospective Randomized Study. Circ. Arrhythmia Electrophysiol. 2017, 10, e005767.
- Chun, J.K.R.; Bordignon, S.; Last, J.; Mayer, L.; Tohoku, S.; Zanchi, S.; Bianchini, L.; Bologna, F.; Nagase, T.; Urbanek, L.; et al. Cryoballoon Versus Laserballoon: Insights From the First Prospective Randomized Balloon Trial in Catheter Ablation of Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2021, 14, e009294.
- Verma, A.; Jiang, C.Y.; Betts, T.R.; Chen, J.; Deisenhofer, I.; Mantovan, R.; Macle, L.; Morillo, C.A.; Haverkamp, W.; Weerasooriya, R.; et al. Approaches to catheter ablation for persistent atrial fibrillation. New Engl. J. Med. 2015, 372, 1812–1822.
- Hussein, A.; Das, M.; Riva, S.; Morgan, M.; Ronayne, C.; Sahni, A.; Shaw, M.; Todd, D.; Hall, M.; Modi, S.; et al. Use of Ablation Index-Guided Ablation Results in High Rates of Durable Pulmonary Vein Isolation and Freedom From Arrhythmia in Persistent Atrial Fibrillation Patients: The PRAISE Study Results. Circ. Arrhythmia Electrophysiol. 2018, 11, e006576.
- Ciconte, G.; Baltogiannis, G.; de Asmundis, C.; Sieira, J.; Conte, G.; Di Giovanni, G.; Saitoh, Y.; Irfan, G.; Mugnai, G.; Hunuk, B.; et al. Circumferential pulmonary vein isolation as index procedure for persistent atrial fibrillation: A comparison between radiofrequency catheter ablation and second-generation cryoballoon ablation. Europace 2015, 17, 559–565.
- Omran, H.; Gutleben, K.J.; Molatta, S.; Fischbach, T.; Wellmann, B.; Horstkotte, D.; Körber, B.; Nölker, G. Second generation cryoballoon ablation for persistent atrial fibrillation: An updated meta-analysis. Clin. Res. Cardiol. 2018, 107, 182–192.
- Liu, X.H.; Gao, X.F.; Jin, C.L.; Chen, C.F.; Chen, B.; Xu, Y.Z. Cryoballoon versus radiofrequency ablation for persistent atrial fibrillation: A systematic review and meta-analysis. Kardiol. Pol. 2020, 78, 20–29.
- Marrouche, N.F.; Wilber, D.; Hindricks, G.; Jais, P.; Akoum, N.; Marchlinski, F.; Kholmovski, E.; Burgon, N.; Hu, N.; Mont, L.; et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: The DECAAF study. JAMA 2014, 311, 498–506.
- Marrouche, N.F.; Greene, T.; Dean, J.M.; Kholmovski, E.G.; Boer, L.M.; Mansour, M.; Calkins, H.; Marchlinski, F.; Wilber, D.; Hindricks, G.; et al. Efficacy of LGE-MRI-guided fibrosis ablation versus conventional catheter ablation of atrial fibrillation: The DECAAF II trial: Study design. J. Cardiovasc. Electrophysiol. 2021, 32, 916–924.
- Lee, J.M.; Shim, J.; Park, J.; Yu, H.T.; Kim, T.H.; Park, J.K.; Uhm, J.S.; Kim, J.B.; Joung, B.; Lee, M.H.; et al. The Electrical Isolation of the Left Atrial Posterior Wall in Catheter Ablation of Persistent Atrial Fibrillation. JACC Clin. Electrophysiol. 2019, 5, 1253–1261.
- Kuck, K.H.; Albenque, J.P.; Chun, K.J.; Fürnkranz, A.; Busch, M.; Elvan, A.; Schlüter, M.; Braegelmann, K.M.; Kueffer, F.J.; Hemingway, L.; et al. Repeat Ablation for Atrial Fibrillation Recurrence Post Cryoballoon or Radiofrequency Ablation in the FIRE AND ICE Trial. Circ. Arrhythmia Electrophysiol. 2019, 12, e007247.
- Aryana, A.; Allen, S.L.; Pujara, D.K.; Bowers, M.R.; O’Neill, P.G.; Yamauchi, Y.; Shigeta, T.; Vierra, E.C.; Okishige, K.; Natale, A. Concomitant Pulmonary Vein and Posterior Wall Isolation Using Cryoballoon With Adjunct Radiofrequency in Persistent Atrial Fibrillation. JACC Clin. Electrophysiol. 2021, 7, 187–196.
- Jiang, X.; Liao, J.; Ling, Z.; Meyer, C.; Sommer, P.; Futyma, P.; Martinek, M.; Schratter, A.; Acou, W.J.; Wang, J.; et al. Adjunctive Left Atrial Posterior Wall Isolation in Treating Atrial Fibrillation: Insight From a Large Secondary Analysis. JACC Clin. Electrophysiol. 2022, 8, 605–618.
- Kamakura, T.; Derval, N.; Duchateau, J.; Denis, A.; Nakashima, T.; Takagi, T.; Ramirez, F.D.; André, C.; Krisai, P.; Nakatani, Y.; et al. Vein of Marshall Ethanol Infusion: Feasibility, Pitfalls, and Complications in Over 700 Patients. Circ. Arrhythmia Electrophysiol. 2021, 14, e010001.
- Nakashima, T.; Pambrun, T.; Vlachos, K.; Goujeau, C.; André, C.; Krisai, P.; Ramirez, F.D.; Kamakura, T.; Takagi, T.; Nakatani, Y.; et al. Impact of Vein of Marshall Ethanol Infusion on Mitral Isthmus Block: Efficacy and Durability. Circ. Arrhythmia Electrophysiol. 2020, 13, e008884.
- Valderrábano, M.; Peterson, L.E.; Swarup, V.; Schurmann, P.A.; Makkar, A.; Doshi, R.N.; DeLurgio, D.; Athill, C.A.; Ellenbogen, K.A.; Natale, A.; et al. Effect of Catheter Ablation With Vein of Marshall Ethanol Infusion vs Catheter Ablation Alone on Persistent Atrial Fibrillation: The VENUS Randomized Clinical Trial. JAMA 2020, 324, 1620–1628.
- Lador, A.; Peterson, L.E.; Swarup, V.; Schurmann, P.A.; Makkar, A.; Doshi, R.N.; DeLurgio, D.; Athill, C.A.; Ellenbogen, K.A.; Natale, A.; et al. Determinants of outcome impact of vein of Marshall ethanol infusion when added to catheter ablation of persistent atrial fibrillation: A secondary analysis of the VENUS randomized clinical trial. Heart Rhythm 2021, 18, 1045–1054.
- Di Biase, L.; Burkhardt, J.D.; Mohanty, P.; Mohanty, S.; Sanchez, J.E.; Trivedi, C.; Güneş, M.; Gökoğlan, Y.; Gianni, C.; Horton, R.P.; et al. Left Atrial Appendage Isolation in Patients With Longstanding Persistent AF Undergoing Catheter Ablation: BELIEF Trial. J. Am. Coll. Cardiol. 2016, 68, 1929–1940.
- Romero, J.; Gabr, M.; Patel, K.; Briceno, D.; Diaz, J.C.; Alviz, I.; Trivedi, C.; Mohanty, S.; Polanco, D.; Della Rocca, D.G.; et al. Efficacy and safety of left atrial appendage electrical isolation during catheter ablation of atrial fibrillation: An updated meta-analysis. Europace 2021, 23, 226–237.
- Tohoku, S.; Chen, S.; Bordignon, S.; Chun, J.K.; Schmidt, B. Hot or cold? Feasibility, safety, and outcome after radiofrequency-guided versus cryoballoon-guided left atrial appendage isolation. J. Arrhythmia 2022, 38, 316–326.
- Chen, S.; Schmidt, B.; Bordignon, S.; Bologna, F.; Lindhoff-Last, E.; Chun, K.R.J. Thrombus Formation in Isolated Left Atrial Appendage After Multiple Atrial Fibrillation Ablations Despite Oral Anticoagulation Followed by Percutaneous Appendage Closure. JACC Clin. Electrophysiol. 2019, 5, 398–400.
- Chen, S.; Schmidt, B.; Tohoku, S.; Trolese, L.; Bordignon, S.; Chun, K.R.J. Transesophageal echocardiography-guided closure of electrically isolated left atrial appendage to constrain a rapidly growing thrombus despite anticoagulation and sinus rhythm. J. Cardiovasc. Electrophysiol. 2020, 31, 247–249.
- DeLurgio, D.B.; Crossen, K.J.; Gill, J.; Blauth, C.; Oza, S.R.; Magnano, A.R.; Mostovych, M.A.; Halkos, M.E.; Tschopp, D.R.; Kerendi, F.; et al. Hybrid Convergent Procedure for the Treatment of Persistent and Long-Standing Persistent Atrial Fibrillation: Results of CONVERGE Clinical Trial. Circ. Arrhythmia Electrophysiol. 2020, 13, e009288.
