Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The knowledge accumulated about liver regeneration has allowed a better understanding of normal liver physiology, by reconstructing the sequence of steps that this organ follows when it must rebuild itself after being injured. The scientific community has used several interdisciplinary approaches searching to improve liver regeneration and, therefore, human health.
- liver regeneration
- compensatory hyperplasia
- interdisciplinary research
- tissue engineering
- decellularization
- xenotransplantation
1. Introduction
Liver regeneration was implicitly recognized in the Greek Prometheus and Tityus myths, but it was Carl von Lagenbunch who performed the first documented successful hepatectomy in humans in 1888 [1][2]. Since then, the interdisciplinary character of scientific progress has been evident. For instance, the observations made by the chemist Louis Pasteur, who for the first time recognized the close relationship between microbes and infectious diseases, were successfully interpreted by the British surgeon Joseph Lister in 1865. This knowledge led him to introduce the use of diluted solutions of carbolic acid as an antiseptic agent [3].
During the period between 1880 and World War II, a big step forward in attaining better survival rates was achieved by the implementation of antiseptic procedures during and after any kind of surgery. After World War II, the main efforts were focused on deepening the knowledge of liver anatomy and the development of different technologies to provide liver support to patients (Figure 1).
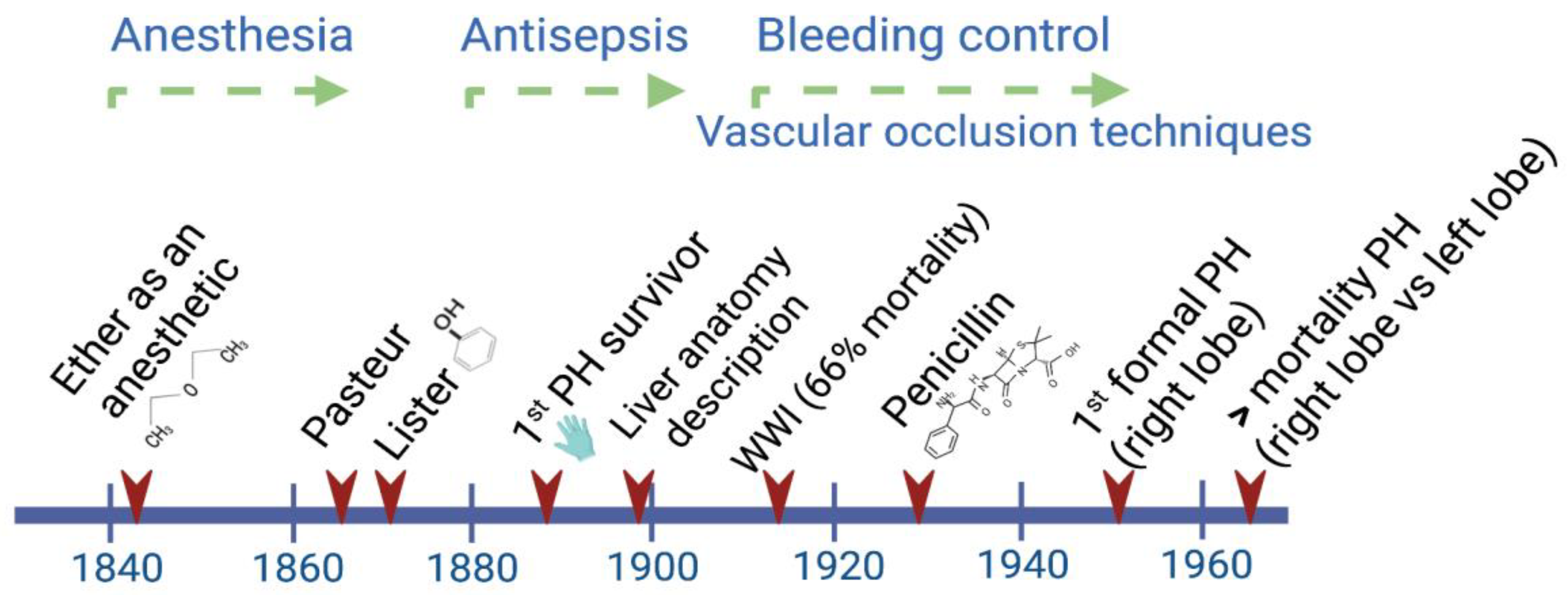
Figure 1. Main advances through the early history of liver surgery have involved interdisciplinary work. By the time Lagenbunch managed to achieve patient survival following a partial hepatectomy (PH), antiseptic procedures including the use of gloves resulted in a greater chance of success. At the beginning of the modern period after World War II, the better control of hemorrhages significantly contributed to a reduction in the mortality rate after right/left liver resections [4].
Unfortunately, in the last few decades, important challenges directly affecting the liver have arisen related to modern lifestyles, such as poor eating habits, low physical activity, and environmental factors, which have skyrocketed the incidence of liver diseases. A possible option to overcome the pace at which liver diseases are increasing is to take advantage of the regeneration capacity the liver inherits, which can only be achieved by gaining a better and deeper understanding of this important process.
2. Interdisciplinary Models Applied to Translational Medicine
2.1. Animal Models of Liver Regeneration
Even though the regenerative capacity of the human liver has been long recognized, the use of animal models has played a key role towards advancing clinical practice. Rat and mouse models are the most widely used due to their size, costs, reproducibility, and ethical advantages.
Most of the knowledge related to liver regeneration in mammals has been obtained from the classical model described in the rat, known as 70% partial hepatectomy (PH), which involves the resection of two thirds of the liver tissue [5]. At that time, although the mechanisms were not understood, it was known that cell division in the hepatic tissue was a rare event that might be reverted by performing a hepatectomy. The first attempts to understand this process came from experiments using the parabiosis approach, where two or three animals shared blood circulation. It was demonstrated that, after performing 70% PH in one of the two partners (or after 80% PH in two of three rats), the intact liver showed high mitosis rates, and an increase in liver weight and in the number of total cells [6][7]. These findings supported the suspected role played by the portal blood flow in the liver and prompted the search for the specific humoral factors involved.
A constant widely documented in mammals is the strict proportionality of around 3% between the liver mass and the individual’s size. For example, when a large dog receives a liver from a smaller dog, the transplanted liver grows to reach the required mass [8]. Accordingly, liver size increased after a patient was transplanted with the liver of a baboon, although he only survived for 70 days [9]. This observation led to the concept of “hepatostat” in reference to the programmed stability of the liver size that is maintained by the complex sensing and signaling molecular toolkit that starts a few minutes after a liver section is resected and continues until the original liver mass is recovered [10]. Therefore, liver regeneration is now strictly conceived as a compensatory hyperplasia process where the lost mass is replaced by the remaining tissue.
Regarding the mechanisms involved in the process of liver mass recovery, Higgins and Anderson observed an increase in cell size (hypertrophy) as an early response followed by mitosis two or three days after PH [5]. Later, these processes were further studied in mice, and now it is known that hypertrophy alone may explain regeneration when 30% of the liver is resected; in contrast, when 70% PH is performed, hypertrophy and the increase in the number of cells (hyperplasia) are involved [11][12].
One of the main contributions provided by research in small species has been the use of transgenic and knockout mouse models; this topic has been extensively studied by Fausto’s group and recently reviewed by other research groups [13][14][15]. Together, these studies have shown that physiological factors such as cytokines and other signaling molecules regulate liver regeneration. It has also been demonstrated that when some of the genes regulating these factors are knocked out, liver regeneration still progresses although at a lower rate, indicating the compensatory mechanisms performed by the diverse molecules.
In addition, by using lineage-tracing techniques and the analysis of functional or metabolic markers, it is now known which specific population of hepatocytes contributes to hepatic renewal after PH, and the possible origin of cells differentiating into hepatocytes [16][17][18]. Interestingly, the distribution of tasks in the liver lobules seems to contribute to proper functioning, even while liver regeneration is taking place.
While the regeneration process is similar between small mammal species and the human [19], larger experimental animals offer easier handling and a closer proximity to human physiology. Therefore, liver resection at different extents has been performed in other species such as dogs [20], pigs [21], and non-human primates [22]. Pigs, with similarities to humans regarding feeding habits, anatomy, and physiology, have become a good model to study liver regeneration, and to explore one of the most common complications following liver resection, which is the development of small-for-size syndrome (SFSS) [23][24][25][26]. This syndrome is triggered by excessive portal venous inflow and the small remnant liver, resulting in risks such as cholestasis, liver insufficiency, and even death.
The mechanisms mediating the regeneration process, after performing these procedures followed by a subsequent hepatectomy, have been an area of intensive research. Despite important hemodynamic differences such as the preservation of the arterial flow in the case of embolization, the mechanisms for liver regeneration between portal vein embolization and partial hepatectomy are believed to be essentially the same [27]. However, there have been some controversies, since based on the observation that the administration of follistatin, an activin inhibitor, did not increase regeneration in non-ligated lobes, it has been suggested that different mechanisms might be involved [28]. What is clearly known is that the hemodynamic changes caused by the alterations in portal flow are related to the hypertrophy of the liver [29].
Together, this knowledge supports the notion that liver regeneration evolved as an adaptive response to liver injury [30]. Regardless of their size, all known vertebrate models have contributed to advance the knowledge related to liver regeneration, starting with the zebrafish, which shares not only a high proportion of orthologous genes with the human, but some of the cell plasticity mechanisms that the liver displays after severe damage [31].
2.2. Models Applied to Predict Different Scenarios after Liver Resection in Humans
The liver receives a double blood supply, 75% coming from the portal vein (deoxygenated blood) and the rest provided by the hepatic artery (oxygenated blood). Once inside the liver, the portal vein and the hepatic artery branch irrigate the lobules that are the functional unit of the liver. The lobules have a polygonal shape, wherein their vertices converge small branches of the portal vein, the hepatic artery, and the bile duct; these three components make the portal triad. The blood flow follows through the sinusoids toward the central vein in the middle of the lobule, while the bile flow runs in the opposite direction through the canaliculi of the hepatocytes.
The liver depends strongly on its vascular system to trigger early responses to stimuli such as 70% PH, after which the remnant liver abruptly receives the blood supply originally needed for a complete liver; thus, the portal flux for the remnant liver is estimated to increase three-fold [32]. Accordingly, the factors promoting liver regeneration coming from the portal vein are also concentrated. Associated with this phenomenon, there are several clinical risks observed in patients after receiving a transplant such as post-hepatectomy liver failure (PHLF) and the activation of small-for-size syndrome (SFSS). Motivated by these concerns, several interdisciplinary groups have worked on developing not only formulas to estimate the remnant liver mass after the partial resection of the liver in humans and pigs [33][34], but also models to understand the normal physiology of the liver vasculature and to predict scenarios in patients after undergoing surgery. Interestingly, based on data obtained from donors that had undergone right lobe hepatectomy, the adverse effects on donor outcomes were estimated to occur when the ratio of remnant liver/total liver volume was ≤30% [35].
The availability of information (e.g., 3D-CT angiographies and computed tomography scans) obtained from the donors and recipients of liver transplants has contributed to make important clinical predictions. For example, the recovery time needed for liver donors has been estimated using a model based on the metabolic load imposed by liver regeneration [36]. Also, another research group interested in preventing post-hepatectomy liver failure validated and reported a model that predicted the probability of success of a liver resection [37].
The vascular network of the human liver has been modeled and it has been determined that all vessels overlap and have a predictable dendritic nature [38][39][40]. This pattern is an efficient system to irrigate the whole lobule, and its configuration has been demonstrated to be optimal at reducing pressure losses. This model was taken further by considering a total number of lobules close to 4.8 × 106, and other parameters associated with each lobule: volume, length of one side of the hexagon, thickness, radius, permeability, and mass flow rates and internal/outer pressures [41]. Then, the authors simulated conditions where several hepatic lobules were “obstructed” to reach theoretical liver resections of 33 or 78%. They observed increases in portal pressure and portal flow, and a decrease in arterial flow with no change of hepatic arterial pressure, mimicking the causes of SFSS [41].
3. Strategies to Face Current Challenges in Clinical Practice
3.1. Extracorporeal Liver Support and Liver Preservation Techniques
Although the liver has a powerful capacity to regenerate, there are several end-stage chronic liver diseases such as cirrhoses of different etiology, hepatocellular carcinomas, and some severe acute liver diseases that go beyond this ability. In these cases, patients may require a liver transplant from a living or a deceased donor. Unfortunately, the number of people needing a liver transplant is far higher than the number of donors [42]. To face these challenges, the scientific community has implemented different interdisciplinary strategies attempting to provide temporal support to patients or long-term solutions to pathologies affecting the liver, and to prevent the risks associated with extended hepatectomies.
Currently, several devices are available that can provide temporal liver support to patients while waiting for a liver transplant (bridging transplantation) [43]. These devices perform the functions of detoxification, regulation, and synthesis, contributing to improve the survival of patients with acute liver failure [44][45][46]. These devices have been tested as a therapeutic bridge while the liver regenerates or while an organ becomes available. These detoxification systems can utilize living cells or are cell-free systems that use albumin mainly as a detoxifying agent [46][47]. There have been contrasting results, with some indicating their benefit while others have shown no benefit [48]. The benefits observed have been mainly related to the improvement in biochemical parameters including bilirubin, ammonia, creatinine levels, and inflammatory cytokines among others, and consequently a benefit in encephalopathy [44][49][50]. Although the benefit in survival has been limited, research in this field continues due to the promising potential these devices have [46].
On the other hand, given the imbalance between the high demand for a liver for transplantation and the actual liver donors, the requirements to donate have been expanded according to the “extended criteria donor”, where age and the presence of fatty liver are no longer used as exclusion parameters. Under these criteria, a few years ago an ex situ procedure consisting in the dynamic preservation of the liver previous to transplantation was adopted [51]. To date, it is possible using a normothermic machine perfusion to preserve the liver for >24 h up to 7 days. This automated machine works at 37 °C and attempts to mimic a more physiological scenario by pumping solutions and employing oxygenating systems recreating what the heart and lungs would do in the body. This machine also removes metabolic waste as the kidneys do, and by adding hormones and nutrients the functions of the intestine and pancreas are mimicked [52]. The use of a hypothermic machine (4–6 °C) has also been shown to reduce very common ischemia-reperfusion injury. Machine perfusion systems have also been used for the testing of different therapeutic components to be added to the perfusate in order to improve liver quality, aiming at increasing transplantation success. However, there are still some issues to be solved such as the development of new viability criteria, as well as the assessment of the safety and efficacy of this method.
3.2. Tissue Engineering Techniques and Biotechnological Advances
Scaffolds made of different biocompatible materials (synthetic or natural), which provide conditions similar to the native extracellular matrix (ECM) of the liver, have helped advance the knowledge of liver regeneration [53][54]. The decellularization of the liver, for instance, provides a scaffold that maintains the native ECM and the hepatic vasculature that could be used in repopulation experiments to study liver regeneration.
The best procedure for whole-liver decellularization uses the perfusion of detergents, enzymes, and chelating agents through the portal vein that solubilize lipids and eliminate cells and nucleic acids. In order to preserve the 3D ultrastructure, composition, and biological activity of the ECM, the procedure needs to be optimized by using different concentrations, combinations of reagents, and time and pressure used for perfusion. After the whole procedure, a washing step to eliminate the decellularization reagents and sterilization with gamma radiation may be needed to reseed the organ. The first attempts to achieve liver decellularization were performed in small species; later, the procedure was reported in pigs, sheep, rabbits, and in humans [55][56][57][58].
There has been some success in humans using this procedure; Mazza et al. performed perfusion of the left lobe over 14 days, while the whole human liver took 6 weeks to be completely decellularized [58]. They reported a good preservation of the liver tissue architecture, where collagen types I, III, IV, and fibronectin were also detected. Importantly, small cubes obtained from the whole decellularized liver were seeded with different human cells (hepatic stellate cells -LX2-, HepG2, and SK-Hep1 cells) that showed growth for 21 days [58]. Additional studies have shown well-preserved ECM by histology, electron microscopy, and proteomic analyses and successful repopulation with mesenchymal stromal cells or endothelial cells from the umbilical vein that restored the vascular lining [59].
The final goal pursued by the decellularization of the liver is to obtain a “bioengineered liver” that can be transplanted and relieve the shortage of organs available for transplantation. Once the liver is cell-free and the ECM is well preserved, a bioengineered liver must accomplish the following properties: a proper vascular permeability to distribute new cells in the right location, reseeded cells that can be of parenchymal (hepatocytes) or non-parenchymal type (e.g., hepatic stellate cells, endothelial cells), and the provision of oxygen and nutrients supporting cell viability. Although scientists have implemented several strategies to solve these issues, to date only short graft survival has been reached [60]. The additional technical problems that are still under intense research are those related to maintaining hepatocytes in a differentiated state. The use of growth factors and culture conditions, including the use of collagen-I, have mildly alleviated this issue. The availability of hepatocytes for their direct transplantation or for the reseeding of the decellularized liver is also still a limitation that has prompted the use of embryonic stem cells. An important contribution made by Takahashi and Yamanaka in 2006 consisted in the use of induced pluripotent stem cells (iPSCs) obtained from reprogrammed mouse embryonic or adult fibroblasts avoiding the immunological rejection associated with stem cells of embryonic origin [61]. This procedure was then successfully reproduced using adult human fibroblasts [62]. Pluripotent stem cells (PSCs) have significantly contributed to improve the understanding of hepatocyte differentiation and this knowledge has been applied towards the development of 3D models, such as spheroids and organoids, aiming at better mimicking the liver structure. Important contributions have been made by Takebe et al., who used different cell types to produce hepatocytes and an arrangement similar to the liver tissue [63][64]. Mun et al. have also shown the formation of hepatocyte-like liver organoids from PSCs including embryonic stem cells and iPSCs. These organoids expressed mature hepatocyte markers and thus the ability to produce proteins such as albumin and metabolized drugs, therefore representing an important tool for toxicological outcome production [65]. Moreover, these organoids allowed their use to test and study the regenerative capacity of the liver after chemical insults such as the administration of acetaminophen, making these models an extremely important tool for studying liver regeneration, modeling liver disease, drug screening, and for personalized medicine [65][66][67][68][69]. With the most recent advances in the field, it is now possible to produce functional liver spheres by the self-aggregation of hepatocytes, endothelial cells, and hepatic stellate cells properly differentiated from human pluripotent stem cells (hPSC) [70]. Moreover, these procedures have been automated with excellent results in terms of functionality, reliability, and reproducibility [70]. Additionally, and importantly, there are currently optimized protocols to obtain hepatocytes from PSCs, reducing the risk of teratoma formation due to the presence of remnant undifferentiated PSCs [71][72]. Interestingly, the known liver zonation associated with the O2 gradients that determine functional and metabolic differences within the liver [73] has also been achieved in the liver spheres, which has supported mathematical models that determine in advance the relevance of performing experimental work [74].
On the other hand, recently, an in vitro model developed to deepen the knowledge of liver physiology in humans was achieved by employing a one-channel microfluidic device. In this model, potential hemodynamic alterations, the cytokines promoting regeneration, and the paracrine communication between hepatocytes and endothelial cells that are involved during liver regeneration were considered [75]. Briefly, spheroids containing primary human hepatocytes and human dermal fibroblasts were resuspended in fibrinogen and thrombin and placed at both sides of the middle channel; this unique channel represented the sinusoid that was embedded with an ECM and where endothelial cells were added. Then, after applying for three days flow alone or flow plus cytokines, the products were collected and analyzed. Interestingly, as a result of applying fluid flow, more than ten cell-derived factors related to liver regeneration were detected; in the presence of cytokines, the hepatocytes also entered into the cell cycle. This approach opens the possibility to explore different combinations of cells, factors, and other conditions, to study the mechanisms affecting liver regeneration in the early stages.
3.3. Liver and Hepatocyte Xenotrasplantation
Liver and hepatocyte xenotransplantation have been explored as therapeutical options for liver diseases and a modality to alleviate the organ supply. Pigs, specifically, have a great advantage in their use as cell or whole organ donors. Physiologically they have some similarities to humans, but can be genetically modified to accomplish a more equal genetic background [76][77][78]. One of the major hurdles that has prevented liver xenotransplantation becoming a widely used procedure has been the lack of long-term survival due to hyperacute rejection [79], platelet and red blood cell destruction [80][81][82], and incompatibilities in the coagulation system [83], leading to severe clotting or bleeding and therefore graft loss. There have also been some ethical issues relating to the use of pigs for transplantation purposes, including the risk of zoonotic pathogen transmission such as the porcine endogenous retroviruses (PERV) [84]. However, the recently documented first pig-to-human organ transplant into a patient providing full support and a 2-month survival demonstrated that xenotransplantation is indeed the future of organ transplantation [85]. Therefore, research in this field should continue to seriously investigate the different hurdles that are delaying its establishment as an alternative therapy to overcome the current organ shortage.
This entry is adapted from the peer-reviewed paper 10.3390/cells11223696
References
- Tiniakos, D.G.; Kandilis, A.; Geller, S.A. Tityus: A forgotten myth of liver regeneration. J. Hepatol. 2010, 53, 357–361.
- Felekouras, E.S.; Kaparelos, D.C.; Papalampros, E. The history of liver surgery, hepatectomy and haemostasis. Hellenic J. Surg. 2010, 82, 280–296.
- Smith, K.A. Louis Pasteur, the father of immunology? Front. Immunol. 2012, 3, 68.
- Brunschwig, A. Hepatic lobectomy for metastatic cancer. Cancer 1963, 16, 277–282.
- Higgins, G.M.; Anderson, R.M. Experimental pathology of the liver. I. Restoration of the liver of the liver of the white rat following partial surgical removal. Arch. Pathol. 1931, 12, 186–202.
- Bucher, N.L.R.; Scott, J.F.; Aub, J.C. Regeneration of the liver in parabiotic rats. Cancer Res. 1951, 11, 457–465.
- Wenneker, A.S.; Sussman, N. Regeneration of liver tissue following partial hepatectomy in parabiotic rats. Proc. Soc. Exp. Biol. Med. 1951, 76, 683–686.
- Francavilla, A.; Ove, P.; Polimeno, L.; Coetzee, M.; Makowka, L.; Barone, D.H.; Thiel, V.; Starzl, T.E. Regulation of liver size and regeneration: Importance in liver transplantation. Transplant. Proc. 1988, 20, 494–497.
- Starzl, T.E.; Fung, J.; Tzakis, A.; Todo, S.; Demetris, A.J.; Marino, I.R.; Doyle, H.; Zeevi, A.; Warty, V.; Michaels, M.; et al. Baboon-to-human liver transplantation. Lancet 1993, 341, 65–71.
- Michalopoulos, G.K. Liver regeneration. J. Cell Physiol. 2007, 213, 286–300.
- Miyaoka, Y.; Ebato, K.; Kato, H.; Arakawa, S.; Shimizu, S.; Miyajima, A. Hypertrophy and unconventional cell division of hepatocytes underlie liver regeneration. Curr. Biol. 2012, 22, 1166–1175.
- Miyaoka, Y.; Miyajima, A. To divide or not to divide: Revisiting liver regeneration. Cell Div. 2013, 8, 8.
- Fausto, N.; Riehle, K. Mechanisms of liver regeneration and their clinical implications. J. Hepatobiliary Pancreat. Surg. 2005, 12, 181–189.
- Huang, W.; Han, N.; Du, L.; Wang, M.; Chen, L.; Tang, H. A narrative review of liver regeneration-from models to molecular basis. Ann. Transl. Med. 2021, 9, 1705.
- Hadjittofi, C.; Feretis, M.; Martin, J.; Harper, S.; Huguet, E. Liver regeneration biology: Implications for liver tumour therapies. W. J. Clin. Oncol. 2021, 12, 1101–1156.
- Wang, B.; Zhao, L.; Fish, M.; Logan, C.Y.; Nusse, R. Self-renewing diploid Axin2+ cells fuel homeostatic renewal of the liver. Nature 2015, 524, 180–185.
- Chen, F.; Jimenez, R.J.; Sharma, K.; Luu, H.Y.; Hsu, B.Y.; Ravindranathan, A.; Stohr, B.A.; Willenbring, H. Broad distribution of hepatocyte proliferation in liver homeostasis and regeneration. Cell Stem Cell 2020, 26, 27–33.
- So, J.; Kim, A.; Lee, S.-H.; Shin, D. Liver progenitor cell-driven liver regeneration. Exp. Mol. Med. 2020, 52, 1230–1238.
- Fausto, N. Liver regeneration: From laboratory to clinic. Liver Transpl. 2001, 7, 835–844.
- Ku, Y.; Tominaga, M.; Sugimoto, T.; Iwakasi, T.; Fukumoto, T.; Takahashi, T.; Suzuki, Y.; Kuroda, Y. Preoperative hepatic venous embolization for partial hepatectomy combined with segmental resection of major hepatic vein. Br. J. Surg. 2002, 89, 63–69.
- Khan, D.; Hickman, R.; Terblanche, J. A porcine model for the study of liver regeneration. J. Investig. Surg. 1988, 1, 139–142.
- Gaglio, P.J.; Baskin, G.; Bohm, R.; Blanchard, J.; Cheng, S.; Dunne, B.; Davidson, J.; Liu, H.; Dash, S. Hepatectomy and laparoscopic-guided liver biopsy in Rhesus Macaques (Macaca mulatta): Novel approach for study of liver regeneration. Comp. Med. 2000, 50, 363–368.
- Khan, D.; Hickman, R.; Terblanche, J.; von Sommoggy, S. Partial hepatectomy and liver regeneration in pigs- the response to different resection sizes. J. Surg. Res. 1988, 45, 176–180.
- Iguchi, K.; Hatano, E.; Yamanaka, K.; Sato, M.; Yamamoto, G.; Kasai, Y.; Okamoto, T.; Okuno, M.; Taura, K.; Fukumoto, K.; et al. Hepatoprotective effect or pretreatment with olprinone in a swine partial hepatectomy model. Liver Transpl. 2014, 20, 838–849.
- Darnis, B.; Mohkam, K.; Schmitt, Z.; Ledochowski, S.; Vial, J.-P.; Duperret, S.; Vogt, S.; Demian, C.; Golse, N.; Mezoughi, S.; et al. Subtotal hepatectomy in swine for studying small-for-size syndrome and portal inflow modulation: It is reliable? HPB (Oxford) 2015, 17, 881–888.
- Xu, Y.; Navarro-Alvarez, N.; Yang, C.; Markmann, J.F.; Dong, J. A reliable scoring system after major liver resection in mice. J. Surg. Res. 2016, 204, 75–82.
- Yokoyama, Y.; Nagino, M.; Nimura, Y. Mechanisms of hepatic regeneration following portal vein embolization and partial hepatectomy: A review. World J. Surg. 2007, 31, 367–374.
- Tashiro, S. Mechanism of liver regeneration after liver resection and portal vein embolization (ligation) is different? J. Hepatobiliary Pancreat. Surg. 2009, 16, 292–299.
- Moris, D.; Vernadakis, S.; Papalampros, A.; Vailas, M.; Dimitrokallis, N.; Petrou, A.; Dimitroulis, D. Mechanistic insights of rapid liver regeneration after associating liver partition and portal vein ligation for stage hepatectomy. World J. Gastroenterol. 2016, 22, 7613–7624.
- Cox, A.G.; Goessling, W. The lure of zebrafish in liver research: Regulation of hepatic growth in development and regeneration. Curr. Opin. Genet. Dev. 2015, 32, 153–161.
- Delgado-Coello, B. Liver regeneration observed across the different classes of vertebrates from an evolutionary perspective. Heliyon 2021, 7, e06449.
- Michalopoulos, G.K. Liver regeneration after partial hepatectomy. Critical analysis of mechanistic dilemmas. Am. J. Pathol. 2010, 176, 2–13.
- Martínez de la Maza, L.; Prado, V.; Hessheimer, A.J.; Muñoz, J.; García-Valdecasas, J.C.; Fondevila, C.A. novel and simple formula to predict liver mass in porcine experimental models. Sci. Rep. 2019, 9, 12459.
- Christ, B.; Collatz, M.; Dahmen, U.; Herrmann, K.-H.; Höpfl, S.; König, M.; Lambers, L.; Marz, M.; Meyer, D.; Radde, N.; et al. Hepatectomy-induced alterations in hepatic perfusion and function- toward multi-scale computational modeling for a better prediction of post-hepatectomy liver function. Front. Physiol. 2021, 12, 733868.
- Yaprak, O.; Guler, N.; Altaca, G.; Dayanga, M.; Demirbas, T.; Akyildiz, M.; Ulusoy, L.; Tokat, Y.; Yuzer, Y. Ratio of remnant to total liver volume or remnant to body weight: Which one is more predictive on donor outcomes? HPB (Oxford) 2012, 14, 476–482.
- Periwal, V.; Gaillard, J.R.; Needleman, L.; Doria, R. Mathematical model of liver regeneration in human live donors. J. Cell Physiol. 2014, 229, 599–606.
- Yamamoto, K.N.; Ishii, M.; Inoue, Y.; Hirokawa, F.; MacArthur, B.D.; Nakamura, A.; Haeno, H.; Uchiyama, K. Prediction of postoperative liver regeneration from clinical information using a data-led mathematical model. Sci. Rep. 2016, 6, 34214.
- Debbaut, C.; Segers, P.; Cornillie, P.; Casteleyn, C.; Dierick, M.; Laleman, W.; Monbaliu, D. Analyzing the human liver vascular architecture by combining vascular corrosion casting and micro-CT scanning: A feasibility study. J. Anat. 2014, 224, 509–517.
- Ma, R.; Hunter, P.; Cousins, W.; Ho, H.; Bartlett, A.; Safaei, S. Anatomically based simulation of hepatic perfusion in the human liver. Int. J. Numer. Method. Biomed. Eng. 2019, 35, e3229.
- Lorente, S.; Hautefeuille, M.; Sanchez-Cedillo, A. The liver, a functionalized vascular structure. Sci. Rep. 2020, 10, 16194.
- Torres Rojas, A.M.; Lorente, S.; Hautefeuille, M.; Sanchez-Cedillo, A. Hierarchical modeling of the liver vascular system. Front. Physiol. 2021, 12, 733165.
- Mayo Clinic Website. Available online: https://www.mayoclinic.org/tests-procedures/liver-transplant/about/pac-20384842 (accessed on 18 July 2022).
- Struecker, B.; Raschzok, N.; Sauer, I.M. Liver support strategies: Cutting-edge technologies. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 166–176.
- Kanjo, A.; Ocskay, K.; Gede, N.; Kiss, S.; Szakács, Z.; Párniczky, M.S.; Stange, J.; Hegyi, P.; Molnár, Z. Efficacy and safety of liver support devices in acute and hyperacute liver failure: A systematic review and network meta-analysis. Sci. Rep. 2021, 11, 4189.
- Ocskay, K.; Kanjo, A.; Gede, N.; Szakács, Z.; Pár, G.; Eross, B.; Stange, J.; Mitzner, S.; Hegyi, P.; Molnár, Z. Uncertainty in the impact of liver support systems in acute-on-chronic liver failure: A systematic review and network meta-analysis. Ann. Intensive Care 2021, 11, 10.
- Saliba, F.; Bañares, R.; Larsen, F.S.; Wilmer, A.; Parés, A.; Mitzner, S.; Stange, J.; Fuhrmann, V.; Gilg, S.; Hassanein, T.; et al. Artificial liver support in patients with liver failure: A modified DELPHI consensus of international experts. Intensive Care Med. 2022, 48, 1352–1367.
- Narayanan, M.; Vora, R.S.; Flynn, M.M.; Subramanian, R.S. The efficacy of albumin dialysis in the treatment of severe cholestatic drug-induced liver injury. Crit. Care Explor. 2022, 4, e0752.
- Ocskay, K.; Tomescu, D.; Faltlhauser, A.; Jacob, D.; Friesecke, S.; Malbrain, M.; Kogelmann, K.; Bogdanski, R.; Bach, F.; Fritz, H.; et al. Hemoadsorption in ‘liver indication’—Analysis of 109 patients’ data from the CytoSorb International Registry. J. Clin. Med. 2021, 10, 5182.
- Kaps, L.; Schleicher, E.M.; Medina Montano, C.; Bros, M.; Gairing, S.J.; Ahlbrand, C.J.; Michel, M.; Klimpke, P.; Kremer, W.M.; Holtz, S.; et al. Influence of Advanced Organ Support (ADVOS) on cytokine levels in patients with acute-on-chronic liver failure (ACLF). J. Clin. Med. 2022, 11, 2782.
- Klammt, S.; Mitzner, S.R.; Stange, J.; Loock, J.; Heemann, U.; Emmrich, J.; Reisinger, E.C.; Schmidt, R. Improvement of impaired albumin binding capacity in acute-on-chronic liver failure by albumin dialysis. Liver Transpl. 2008, 14, 1333–1339.
- Chedid, M.F.; Pinto, M.A.; Juchem, J.F.G.; Grezzana-Fljo, T.J.M.; Kruel, C.R.P. Liver preservation prior to transplantation: Past, present, and future. World J. Gastroint. Surg. 2019, 11, 122–125.
- Lascaris, B.; de Meijer, V.E.; Porte, R.J. Liver machine perfusion as a dynamic platform for regenerative purposes: What does the future have in store for us? J. Hepatol. 2022, 77, 825–836.
- Khajavi, M.; Hashemi, M.; Kalalinia, F. Recent advances in optimization of liver decellularization procedures used for liver regeneration. Life Sci. 2021, 281, 119801.
- Vazirzadeh, M.; Azarpira, N.; Davoodi, P.; Vosough, M.; Ghaedi, K. Natural scaffolds used for liver regeneration: A narrative update. Stem Cell Rev. Rep. 2022, 18, 2262–2278.
- Uygun, B.E.; Soto-Gutierrez, A.; Yagi, H.; Izamis, M.L.; Guzzardi, M.A.; Shulman, C.; Milwid, J.; Kobayashi, N.; Tilles, A.; Berthiaume, F.; et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. 2010, 16, 814–820.
- Yagi, H.; Fukumitsu, K.; Fukuda, K.; Kitago, M.; Shinoda, M.; Obara, H.; Itanu, O.; Kawachi, S.; Tanabe, M.; Coudriet, G.M.; et al. Human- scale whole-organ bioengineering for liver transplantation: A regenerative medicine approach. Cell Transplant. 2013, 22, 231–242.
- Kajbafzadeh, A.M.; Javan-Farazmand, N.; Monajemzadeh, M.; Baghayee, A. Determining the optimal decellularization and sterilization protocol for preparing a tissue scaffold of a human- sized liver tissue. Tissue Eng. Part C Methods. 2013, 19, 642–651.
- Mazza, G.; Rombouts, K.; Rennie Hall, A.; Urbani, L.; Vinh Luong, T.; Al-Akkad, W.; Longato, L.; Brown, D.; Maghsoudlou, P.; Dhillon, A.P.; et al. Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation. Sci. Rep. 2015, 5, 13079.
- Verstegen, M.M.A.; Willemse, J.; van den Hoek, S.; Kremers, G.-J.; Luider, T.M.; van Huizen, N.A.; Willemssen, F.E.J.A.; Metselaar, H.J.; Ijzermans, J.N.M.; van der Laan, L.J.W.; et al. Decellularization of whole human liver grafts using controlled perfusion for transplantable organ bioscaffolds. Stem Cells Dev. 2017, 26, 1304–1315.
- Li, K.; Tharwat, M.; Larson, E.L.; Felgendreff, P.; Hosseiniasl, S.M.; Rmilah, A.A.; Safwat, K.; Ross, J.J.; Nyberg, S.L. Re-endothelialization of decellularized liver scaffolds: A step for bioengineered liver transplantation. Front. Bioeng. Biotechnol. 2022, 10, 833163.
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultured by defined factors. Cell 2006, 126, 663–676.
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872.
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.-R.; Ueno, Y.; Zheng, Y.-W.; Koike, N.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484.
- Takebe, T.; Sekine, K.; Kimura, M.; Yoshikawa, E.; Ayano, S.; Koido, M.; Funayama, S.; Nakanishi, N.; Hisai, T.; Kobayashi, T.; et al. Massive and reproducible production of liver buds entirely from human pluripotent stem cells. Cell Rep. 2017, 21, 2661–2670.
- Mun, S.J.; Ryu, J.-S.; Lee, M.-O.; Son, Y.S.; Oh, S.J.; Cho, H.-S.; Son, M.-Y.-; Kim, D.-S.; Kim, S.J.; Yoo, H.J.; et al. Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J. Hepatol. 2019, 71, 970–985.
- Kruitwagen, H.S.; Oosterhoff, L.A.; Vernooij, I.G.W.H.; Schrall, I.M.; van Wolferen, M.E.; Bannink, F.; Roesch, C.; van Uden, L.; Molenaar, M.R.; Helms, J.B.; et al. Long-term adult feline liver organoid cultures for disease modeling of hepatic steatosis. Stem Cell Rep. 2017, 8, 822–830.
- Lo Nigro, A.; Gallo, A.; Bulati, M.; Vitale, G.; Paini, D.S.; Pampalone, M.; Galvagno, D.; Conaldi, P.G.; Miceli, V. Amnion-derived mesenchymal stromal/stem cell paracrine signals potentiate human liver organoid differentiation: Translational implications for liver regeneration. Front. Med. 2021, 8, 746298.
- Harrison, S.P.; Baumgarten, S.F.; Verma, R.; Lunov, O.; Dajneka, A.; Sullivan, G.J. Liver organoids: Recent developments, limitations and potential. Front. Med. 2021, 8, 574047.
- Telles-Silva, K.A.; Pacheco, L.; Komatsu, S.; Chianca, F.; Caires-Júnior, L.C.; Silva Araujo, B.H.; Goulart, E.; Zatz, M. Applied hepatic bioengeering: Modeling the human liver using organoid and liver-on-a-chip technologies. Front. Bioeng. Biotechnol. 2022, 10, 845360.
- Lucendo-Villarin, B.; Meseguer-Ripolles, J.; Drew, J.; Fischer, L.; Ma, E.; Flint, O.; Simpson, K.J.; Machesky, L.M.; Mountford, J.C.; Hay, D.C. Development of a cost-effective automated platform to produce human liver spheroids for basic and applied research. Biofabrication 2021, 13, 015009.
- Subba Rao, M.; Sasikala, M.; Reddy, D.N. Thinking outside the liver: Induced pluripotent stem cells for hepatic applications. World J. Gastroenterol. 2013, 19, 3385–3396.
- Kumar, S.; Curran, J.E.; Williams-Blangero, S.; Blangero, J. Efficient generation of functional hepatocytes from human induced pluripotent stem cells for disease modeling and disease gene discovery. Methods Mol. Biol. 2022, 2549, 85–101.
- Kietzman, T. Metabolic zonation of the liver: The oxygen gradient revisited. Redox Biol. 2017, 11, 622–630.
- Leedale, J.A.; Lucendo-Villarin, B.; Meseguer-Ripolles, J.; Kasarinaite, A.; Webb, S.D.; Hay, D.C. Mathematical modelling of oxygen gradients in stem cell-derived liver tissue. PLoS ONE 2021, 16, e0244070.
- Chhabra, A.; Greco Song, H.-H.; Grselak, K.A.; Polacheck, W.J.; Fleming, H.E.; Chen, C.S.; Bhatia, S.N. A vascularized model of the human liver mimics regenerative responses. Proc. Natl. Acad. Sci. USA. 2022, 119, e2115867119.
- Ahrens, H.E.; Petersen, B.; Herrmann, D.; Lucas-Hahn, A.; Hassel, P.; Ziegler, M.; Kues, W.A.; Baulain, U.; Baars, W.; Schwinzer, R.; et al. siRNA mediated knockdown of tissue factor expression in pigs for xenotransplantation. Am. J. Transplant. 2015, 15, 1407–1414.
- Ramirez, P.; Montoya, M.J.; Ríos, A.; García Palenciano, C.; Majado, M.; Chávez, R.; Muñoz, A.; Fernández, O.M.; Sánchez, A.; Segura, B.; et al. Prevention of hyperacute rejection in a model of orthotopic liver xenotransplantation from pig to baboon using polytransgenic pig livers (CD55, CD59, and H-transferase). Transplant. Proc. 2005, 37, 4103–4106.
- Kolber-Simonds, D.; Lai, L.; Watt, S.R.; Denaro, M.; Arn, S.; Augenstein, M.L.; Betthauser, J.; Carter, D.B.; Greenstein, J.L.; Hao, Y.; et al. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc. Natl. Acad. Sci. USA 2004, 101, 7335–7340.
- Kobayashi, T.; Cooper, D.K. Anti-Gal, alpha-Gal epitopes, and xenotransplantation. Subcell. Biochem. 1999, 32, 229–257.
- Kim, K.; Schuetz, C.; Elias, N.; Wamala, I.; Varma, M.; Smith, R.N.; Robson, S.C.; Cosimi, A.B.; Sachs, D.H.; Hertl, M. Up to 9-day survival and control of thrombocytopenia following alpha1,3-galactosyl transferase knockout swine liver xenotransplantation in baboons. Xenotransplantation 2012, 19, 256–264.
- Yeh, H.; Machaidze, Z.; Wamala, I.; Fraser, J.W.; Navarro-Alvarez, N.; Kim, K.; Schuetz, C.; Shi, S.; Zhu, A.; Hertl, M.; et al. Increased transfusion-free survival following auxiliary pig liver xenotransplantation. Xenotransplantation 2014, 21, 454–464.
- Peng, Q.; Yeh, H.; Wei, L.; Enjyoj, K.; Machaidze, Z.; Csizmad, E.; Schuetz, C.; Lee, K.M.; Deng, S.; Robson, S.C.; et al. Mechanisms of xenogeneic baboon platelet aggregation and phagocytosis by porcine liver sinusoidal endothelial cells. PLoS ONE 2012, 7, e47273.
- Navarro-Alvarez, N.; Shah, J.A.; Zhu, A.; Ligocka, J.; Yeh, H.; Elias, N.; Rosales, I.; Colvin, R.; Cosimi, A.B.; Markmann, J.F.; et al. The Effects of exogenous administration of human coagulation factors following pig-to-baboon liver xenotransplantation. Am. J. Transplant. 2016, 16, 1715–1725.
- Denner, J. Why was PERV not transmitted during preclinical and clinical xenotransplantation trials and after inoculation of animals? Retrovirology 2018, 15, 28.
- Wang, W.; He, W.; Ruan, Y.; Geng, Q. First pig-to-human heart transplantation. Innovation (Camb) 2022, 3, 100223.
This entry is offline, you can click here to edit this entry!
