Due to the combination of thermal engineering and the rapid rise of nanotechnology research over the past two decades, novel heat transfer fluids known as “nanofluids” have emerged. A “nanofluid” is a heat transfer fluid that has 1–100 nm-sized “nanoparticles”, which are suspended nanoparticles, scattered throughout the base fluid. To increase the stability of the working fluid, it is crucial to make sure the nanoparticle size is smaller than 100 nm. Water, oils, organic liquids (such as tri-ethylene-glycols, ethylene and refrigerants) and bio-fluids polymeric solutions are the most often utilized base fluids. Numerous studies throughout the years have documented diverse nanofluid preparation methods with various nanoparticle types and their heat transfer capabilities, in addition to advancing the information about nanofluids.
1. Nanofluid Preparation Methods
Different methods of nanofluid preparation yield different thermophysical properties of nanofluids, including stability and thermal conductivity [
12]. The preparation methods can also determine the particle size in the nanofluid, i.e., whether it is a micro-size or nano-size suspension, which will affect the stability of the nanofluid prepared [
13]. In addition, sonication time also plays a vital role in determining the thermophysical properties of the nanofluid prepared, where an increase in the ultrasonication time and power leads to higher heat transfer enhancement, higher thermal conductivity, lower pressure drops and lower viscosity [
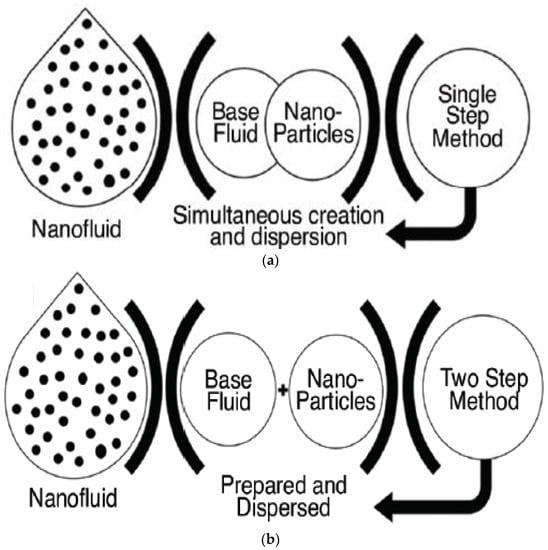
14]. In general, there are two commonly used technique to prepare nanofluids, which are the single-step and the two-step methods.
Table 2 shows the differences and a comparison between the two approaches. Regardless, both methods of preparation obtain homogeneous dispersed nanofluids that contain desirable properties and stable characteristics [
15].
Table 2. Summary of differences between one-step and two-step methods.
| Particular |
One-Step Method |
Two-Step Method |
| Synthesis process |
Simultaneous production of nanoparticles and nanofluid |
Production of nanoparticles either chemically or mechanically followed by dispersion of nanoparticles into the base fluid |
| Production scale |
Small scale production |
Large scale production |
| Cost of production |
High cost |
Low cost |
| Control on NPs size |
Difficult and limited control over the nanoparticle size during the preparation stage |
Able to control the nanoparticle size during the preparation stage |
| Particle oxidation |
Oxidation of particles does not occur due to the elimination of drying, transportation and storage processes. |
No such problem |
| Advantages |
Reduced chances of particle agglomeration.
More stable nanofluid |
Cheaper and more applicable in industry |
| Disadvantages |
Residual reactants are left in the nanofluids which might cause problems during application.
Can only produce in batch |
Prone to agglomeration
Constant stabilizing process is needed for long term stability |
Figure 3. Nanofluid preparation methods (
a) single-step method (
b) two-step method. “Reprinted with permission from Ref [
19]. Copyright 2018 MDPI”.
Table 3. Summary of preparation methods and relative surfactant used in past studies.
| Nanoparticles |
Base Fluid |
Preparation Method |
Nanofluid Sonication Time |
Surfactant |
Reference |
| Metal Based |
|
|
|
|
|
| Au |
Water |
Two step |
20 min |
None |
[20] |
Au
Ag |
DI Water |
One step |
- |
Cationic gemini |
[21] |
| Cu |
Methanol |
Two step |
30 min |
APTMS |
[22] |
| Metal-oxide Based |
|
|
|
|
|
| TiO2 |
Water |
Two step |
5 h |
HMDS |
[23] |
| CuO |
Water |
Two step |
5 h |
None |
[24] |
| Al2O3 |
DI Water |
One step |
- |
None |
[25] |
| Al2O3 |
Water |
Two step |
6 h |
None |
[26] |
| ZnO |
EG |
Two step |
3 h |
None |
[27] |
| Carbon Based |
|
|
|
|
|
| MWCNT |
Water |
Two step |
20 min |
None |
[20] |
| COOH-CNT |
DI Water |
Two step |
10 min |
Nonylphenol ethoxylate |
[28] |
| CNT |
Decane |
Two step |
60 min |
Oleylamine |
[29] |
| MWCNT |
Water |
Two step |
3 h |
SDBS |
[30] |
| MWCNT |
Kapok seed oil |
One step |
6 h |
none |
[31] |
| Hybrid |
|
|
|
|
|
| Ag-MWCNT (50:50) |
DI Water |
Two step |
30 min |
SDBS |
[18] |
| MgO-SWCNT (80:20) |
EG |
Two step |
6 h |
None |
[32] |
Cu-TiO2
(36:64) |
EG/Water (50:50) |
Two step |
30 min |
PVP, SDBS and GA |
[33] |
| ZnO-SWCNT (70:30) |
EG/Water (40:60) |
Two step |
7 h |
None |
[34] |
| ZnO-MWCNT (50:50) |
EG/Water (50:50) |
Two Step |
3 h |
None |
[35] |
Au-TiO2
Au-Ag
Au-Al
Au-Ni |
DI Water |
Two Step |
3 h |
None |
[36] |
Ag-Fe3O4
(50:50) |
DI Water |
Two Step |
3 h |
None |
[37] |
The classification of nanofluids is based on the type of nanoparticles chosen for nanofluid production. In general, the nanofluids can be classified into four different groups, which are (1) metal-based nanofluids, (2) metal oxide-based nanofluids, (3) carbon-based nanofluids or (4) mixed/hybrid metal-based nanofluids. The nanoparticles selected were suspended into base fluids such as oil, water or ethylene glycol. Stability of the nanofluid is crucial as it will affect the thermophysical properties if agglomeration were to occur. Hence, both physical property enhancement and stability of the nanofluid must be taken into consideration during selection of the nanofluids application. The following sections summarize each type of nanofluid described by researchers in the literature.
2.1. Metal-Based Nanofluids
Metal-based nanofluids are prepared by suspending metal nanoparticles such as gold, silver, aluminium, etc., in a base fluid. Beicker et al. [
20] produced a gold/water nanofluid though the two-step method to study the photothermal conversion behavior of the prepared nanofluid. The investigation found that the gold nanofluid was remarkably effective even at a low concentration of 0.004% (volumetric). The prepared nanofluid was recorded to be stable for up to 120 h.
2.2. Metal Oxide-Based Nanofluids
The reasons behind the extensive utilization and widespread industrial applicability of metal oxide-based nanofluids are due to the high stability, suitable thermal conductivity, low cost of the nanoparticles and so forth. Due to its low cost in synthesis, it provides an economical alternative for industry. Hence, many engineering applications utilize metal oxide nanofluids as the cooling medium. Among the metal oxide nanoparticles, titania (TiO2) and alumina (Al2O3) are the most commonly used nanoparticles to synthesis metal oxide-based nanofluids.
2.3. Carbon-Based Nanofluids
The majority of the articles on carbon-based nanofluids reported significant improvement in thermal –physical properties when compared to based fluid. However, the main drawback of carbon-based nanoparticles is their high cost production which limits widespread commercial use. Beicker et al. [
20] studied both metal-based and carbon-based nanofluids and concluded that the MWCNT/water nanofluid would be a better economical choice compared to the gold/water nanofluid. This is because the quality of the gold/water nanofluid degrades faster and had a lower stability when compared to the MWCN/water nanofluid. Carbon-based nanofluids tend to have higher stability according to reports, as shown in
Table 3. Sarafraz et al. [
28] reported 504 h of stability for COOH-CNT/Water nanofluid using Nonylphenol ethoxylate (Steric) as a stabilizer, while Xie et al. [
29] reported stability of up to 1440 h (2 months) for Treated CNTs nanofluid dispersed in both distilled water and ethylene glycol without adding any surfactant. However, nonpolar base fluids such as decene required a small amount of oleylamine (surfactant) for the TCNTs/decene suspensions to remain stable for months.
2.4. Hybrid Nanofluids
Since nanofluids have consistently produced positive potential applications over time, scientists have begun to consider mixing various nanoparticles into base fluids to create what are now known as “hybrid nanofluids.” A hybrid material is something that can concurrently combine the chemical and physical properties of two or more separate materials at the molecular or nanoscale level, and it can deliver these properties in a homogenous state. The hybrid nanofluid was used primarily to obtain the properties of its constituent materials. This is because no single substance had all the necessary features to be effective for a given application. When compared to individual nanofluids, this emerging class of nanofluids shows a considerable improvement in terms of hydrodynamic properties, thermophysical properties and heat transport characteristics. Such improvement was observed in research conducted by Sun et al. [
18], who applied a hybrid nanofluid containing the Ag-multiwall carbon nanotube nanoparticles at 50:50 ratio in a jet impinging cooling system. Results demonstrated that the Ag-MWCNT/water hybrid nanofluid was far more superior over the single-phased MWCNT/water nanofluid in terms of thermal conductivity. The outcomes showed that the thermal conductivity of the Ag-MWCNT/water hybrid nanofluid enhanced significantly when compared to the MWCNT/water nanofluid. Common nanofluid preparation methods such as the one- and two-step methods are used for hybrid-based nanofluid production. An overview of the general nanofluid preparation methods is given in the following section.
3. Nanofluid Stabilization Methods
It is crucial to ensure that nanofluids stability is achieved during the preparation stage to obtain optimal and equal thermophysical properties throughout the applications. A high stability nanofluid is attained when the Electrical Double Layer Repulsive Force (EDLRF) is higher than the Van der Waals force of attraction. If a higher Van der Waals force of attraction between suspended nanoparticles occurs, the agglomeration and aggregation process begins to take place, which results in clustering of the nanoparticles, which eventually leads to sedimentation over time [
38]. Hence, it is crucial that the prepared nanofluid, especially if prepared through a two-step method, undergoes a stability enhancement process before it is applied to any engineering applications.
Table 4 shows a summary of the nanofluid stability period, as detailed by researchers, and the properties of nanoparticles used. It can be observed that carbon-based nanofluids have the highest stability compared to other types of nanofluid. It also can be seen that preparation of metal-based nanofluids using the two-step method with appropriate surfactant can produce stable nanofluids. The following section discusses the techniques utilized in the two-step method for enhancing the stability of the nanofluid.
Table 4. Stability period along with nanoparticles properties from past studies.
| Nanofluid |
Particle Size (nm) |
Concentration |
Stability Period Reported |
References |
| Metal Based |
|
|
|
|
| Au/Water |
10–30 |
0.0001–0.004 vol% |
>120 h |
[20] |
Au/DI Water
Ag/DI Water |
8.6–9.4
4–33 |
- |
80 h |
[21] |
| Cu/Methanol |
25–75 |
0.1–10 wt% |
4320 h |
[22] |
| Metal-oxide Based |
|
|
|
|
| TiO2/Water |
30–50 |
0.5–2.5 vol% |
168 h |
[23] |
| CuO/Water |
30–50 |
2–4 vol% |
168 h |
[24] |
| Al2O3/DI Water |
20 |
0.05–0.25 kg/m3 |
- |
[25] |
| Al2O3/Water |
30 |
0.5–2 vol% |
480 h |
[26] |
| ZnO/EG |
10–20 |
1–5 vol% |
6 h |
[27] |
| Carbon Based |
|
|
|
|
| MWCNT/Water |
Outer D: 50–80
Inner D: 5–15
L: 10–20 (µm) |
0.0001–0.03 vol% |
<120 h |
[20] |
| COOH-CNT/DI Water |
D: 12–14
L: 1.5–2 (µm) |
0.1–0.3 wt% |
504 h |
[28] |
| CNT/Decane |
D: 15
L: 30 (µm) |
0.1 vol% |
1440 h |
[29] |
| MWCNT/Water |
Outer D: 50–80
Inner D: 5–15
L: 10–20 (µm) |
0.1–0.5 vol% |
1080 h |
[30] |
| MWCNT/Kapok Seed Oil |
D: 15.79–19.21 |
0.1 wt% |
<720 h |
[31] |
| Hybrid |
|
|
|
|
| Ag-MWCNT/DI Water |
Ag: 50
MWCNT: 20–30 |
0.01–0.05 wt% |
48 h |
[18] |
| MgO-SWCNT/EG |
- |
0.05–1 vol% |
- |
[32] |
| Cu-TiO2/EG-Water |
Cu: 40–60
TiO2: <25 |
0.2–0.8 wt% |
- |
[33] |
| ZnO-SWCNT/EG-Water |
ZnO: 10–30
SWCNT –
Outer D: 1–2
Inner D: 0.8–1.6 |
0.05–1.6 vol% |
- |
[34] |
| ZnO-MWCNT/EG-Water |
ZnO: 10–30
MWCNT -
Outer D: 5–15
Inner D: 3–5 |
0.02–1 vol% |
240 h |
[35] |
Au-TiO2/DI Water
Au-Ag/DI Water
Au-Al/DI Water
Au-Ni/DI Water |
Au: 45–85
TiO2: 15–40
Ag:30–65
Al: 50–75
Ni: 25–65 |
0.05–3 vol% |
<168 h |
[36] |
| Ag-Fe3O4/DI Water |
21 |
0.015 |
- |
[37] |
A magnetic stirrer which is also known as magnetic mixer is a device that is widely used in laboratories that contains a stationary electromagnet or rotating magnet. The function is to generate a rotating magnetic field, hence enhancing the homogeneity by decreasing sediment in a nanofluid. This device can be used to make a mixed solution, quickly spin, stir, immerse in a liquid or make a stir bar. Typically, the device has two knobs where the left knob is to control the stirring rate while the right knob is to control the heating rate [
39,
40]. This technique is often used before sonication, especially in hybrid nanofluid preparation, to mix the hybrid nanoparticles before dispersing it into the base fluid [
41].
3.2. Surfactants
The stability of a nanofluid can also be enhanced by introducing compounds known as surfactants or dispersants into the nanofluid. The presence of this compound can lower the surface tension between the nanoparticles and base fluid with the cost of deterioration of the thermophysical properties of nanofluid. This is because surfactants manage to improve the stability by preventing agglomeration and aggregation only when it is used at the optimal quantity, as excess usage may cause degradation of the chemical stability as well as decrease in thermal conductivity of the nanofluid [
42,
43]. The chemical properties of surfactant consist of two main parts, which are the hydrophilic polar head group followed by the long hydrocarbon chain known as hydrophobic tail. Some common surfactants used by researchers include Sodium dodecyl benzene sulfonate (SDBS), Oleic Acid (OA), Arabic gum, Polyvinylpyrrolidone (PVP), etc.
3.3. Sonification
The process of implementing sound energy to agitate the nanoparticles is known as sonication. Nanoparticles that are subjected to sonication experience strong vibration from the ultrasonic waves which are usually higher than 20 kHz. The optimum ultrasonic time for preparation of nanofluids is yet to be fully discovered, but researchers have found that the optimum sonication time depends on the concentration and type of nanoparticles used. Higher concentration of nanoparticles often requires higher optimum sonication time. It has also been found that exceeding the optimum sonication time could decrease the stability period of nanofluid [
44,
45]. This method provides better dispersion when compared to magnetic stirring [
41]. Two types of sonicator that are widely used by researchers to enhance the nanofluid stability are the probe type and bath type. On comparison between the two, it was reported that probe type provides better enhancement and performance when compared to bath type sonication [
46].
This entry is adapted from the peer-reviewed paper 10.3390/mi13122059