Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Quinazoline is an essential scaffold, known to be linked with various biological activities. Some of the prominent biological activities of this system are analgesic, anti-inflammatory, anti-hypertensive, anti-bacterial, anti-diabetic, anti-malarial, sedative–hypnotic, anti-histaminic, anti-cancer, anti-convulsant, anti-tubercular, and anti-viral activities. This diversity in the pharmacological response of the quinazoline system has encouraged medicinal chemists to study and discover this system and its multitude of potential against several biological activities.
- quinazoline
- design
- synthesis
1. Introduction
Quinazoline is a double-ring heterocyclic system with two nitrogen heteroatoms in the six-membered aromatic ring fused to the benzene ring [1][2][3][4]. Quinazoline is formed from the pyrimidine ring fused to the benzene ring at two adjacent carbon atoms (Figure 1). It is classified as phenyl pyrimidine [5][6][7]. The first quinazoline derivative was synthesized by Griess et al. in 1869 through a condensation reaction [8]. It was also prepared from 2-carboxylate derivatives by a decarboxylation reaction [9][10][11][12]. Several quinazoline derivatives were synthesized and studied for their physical and chemical properties in 1903 by Gabriel and Colman [8]. The quinazoline system may contain an oxo group (=O) at C-2 to form the carbonyl group (C=O), named quinazoline-2(1H)-one (2-quinazolinone) 1 (Figure 1), or at C-4 and named quinazoline-4(3H)-one (4-quinazolinone) 2 (Figure 1). Otherwise, it may contain two oxo groups at C-2 and C-4 to form quinazoline-2,4-dione 3 (quinazolinedione) (Figure 1) [13][14][15][16]. Quinazolines are a group of versatile derivatives with a wide range of pharmacological activities [17][18][19][20]. They are used as analgesic, anti-hypertensive, anti-inflammatory, anti-bacterial, anti-diabetic, sedative–hypnotic, anti-histaminic, anti-cancer, anti-convulsant, anti-tubercular, and anti-viral agents, as well as having many other uses [21][22][23][24][25].
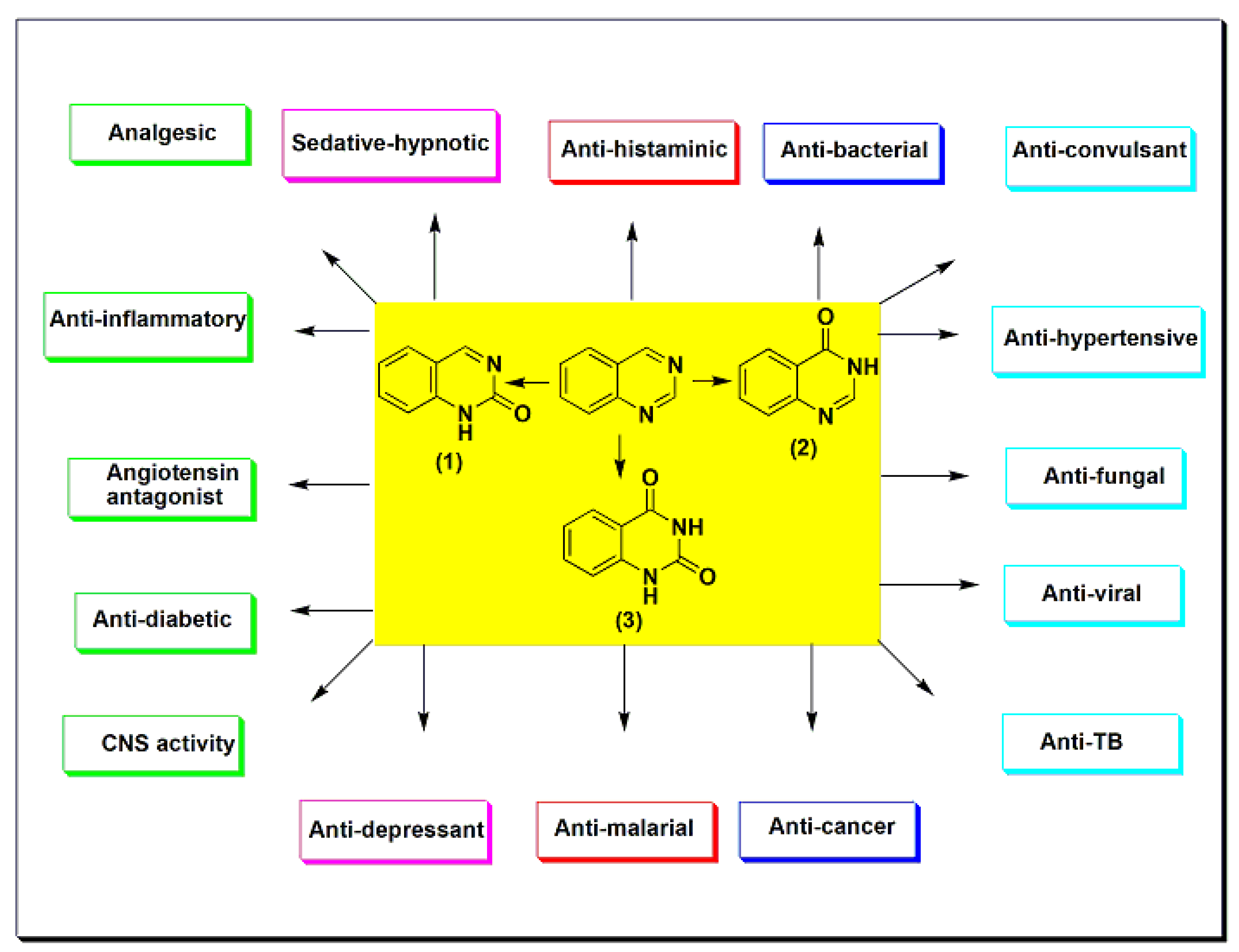
Figure 1. The quinazolines and some of their pharmacological activities.
2. Physicochemical Characters of Quinazolines
Figure 2 shows the molecular structures of quinazoline, 4-quinazolinone, 2-quinazolinone, and 2,4-quinazolinedione, represented by ball-and-line mode [26]. Table 1 shows the physicochemical characters of quinazoline, 2-quinazolinone, 4-quinazolinone, and 2,4-quinazolinedione [27]. Figure 3 and Figure 4 show the lipophilic and the electrostatic potentials of these quinazolines [26].
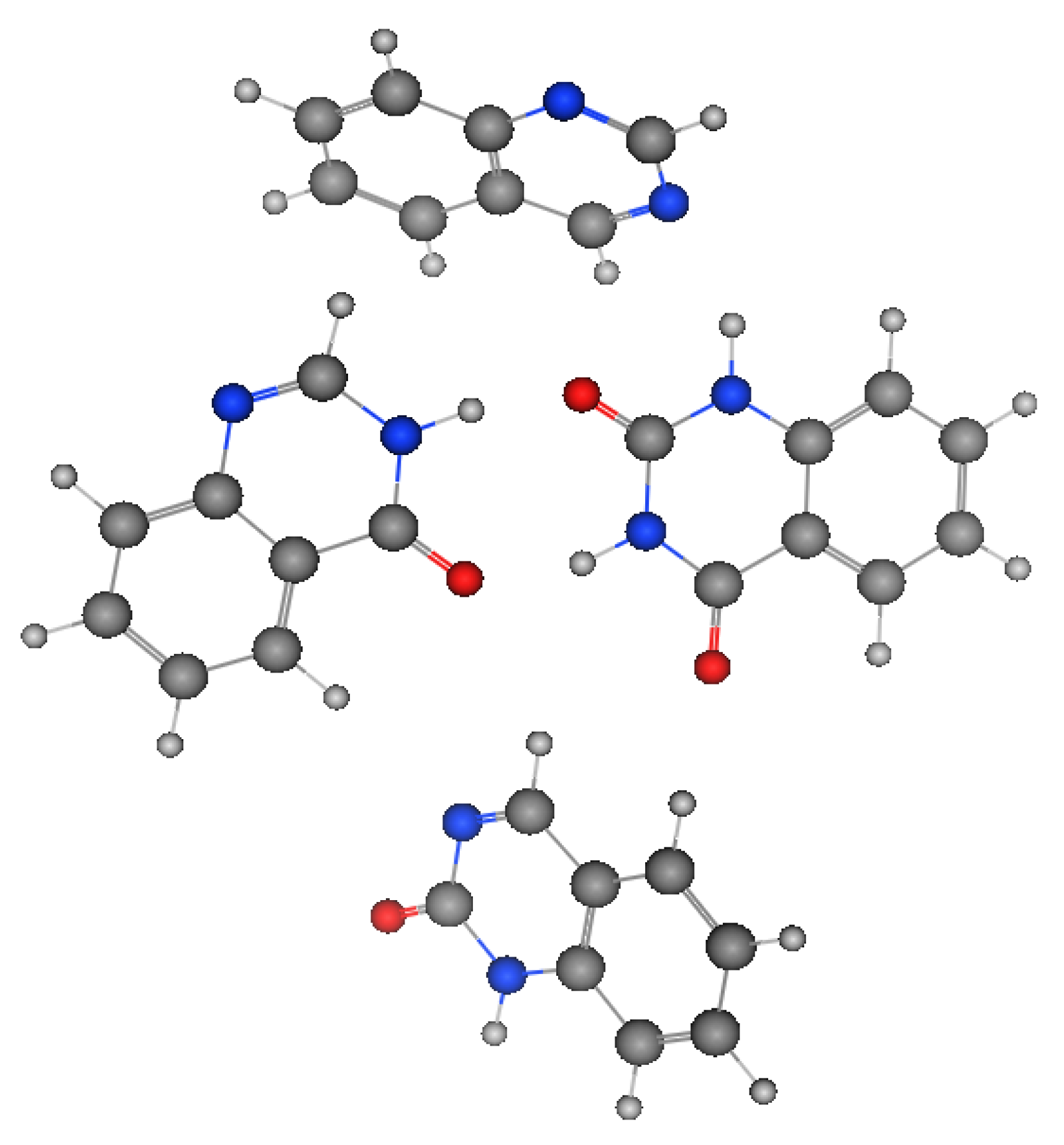
Figure 2. Molecular structure of quinazoline, 2-quinazolinone, 4-quinazolinone, and 2,4-quinazolinedione, represented by ball-and-line mode.
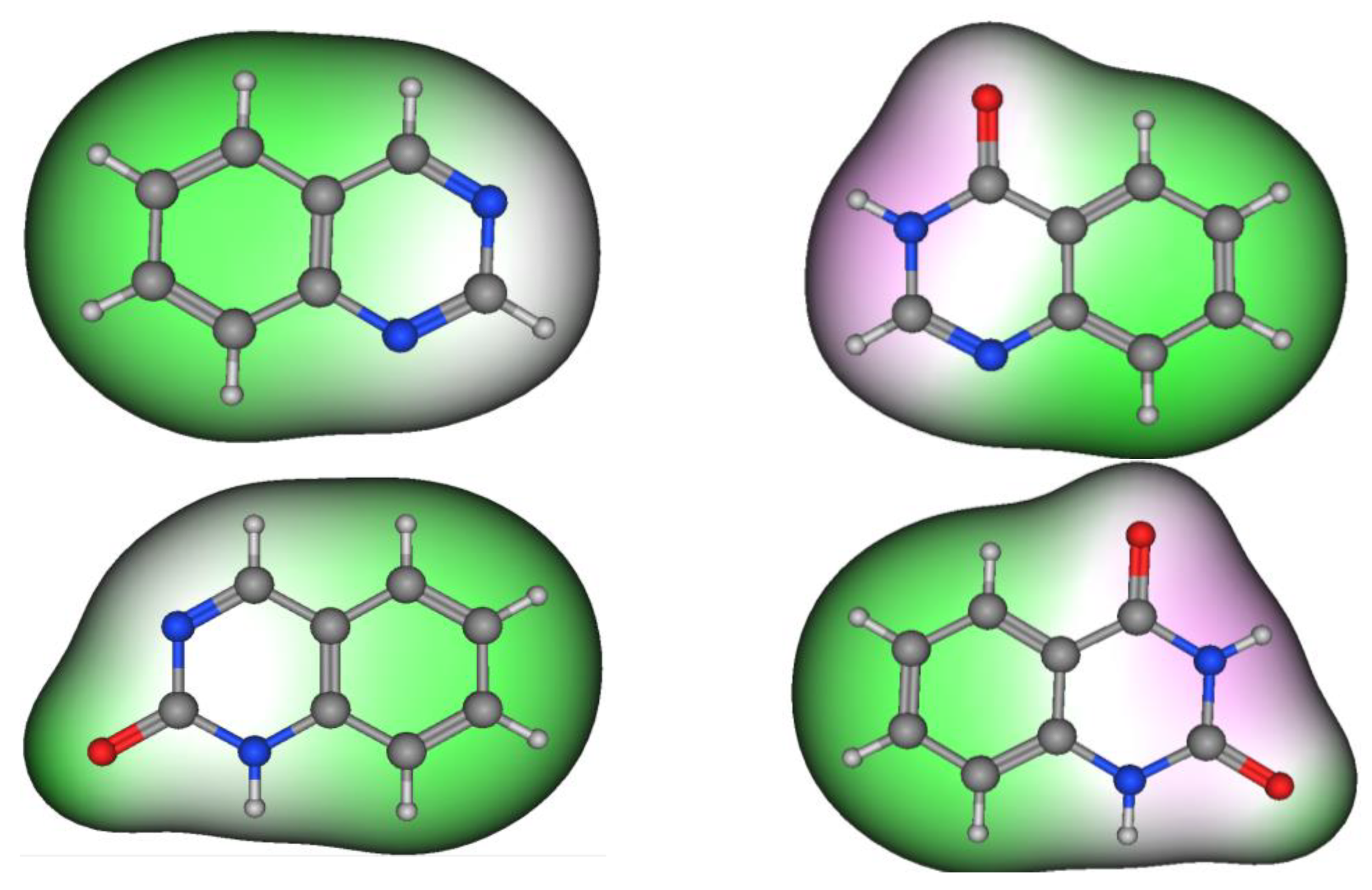
Figure 3. Lipophilic potential of quinazoline, 4-quinazolinone, 2-quinazolinone, and 2,4-quinazolinedione. Green color represents lipophilic area, white color represents neutral area, and pink color represents hydrophilic area.
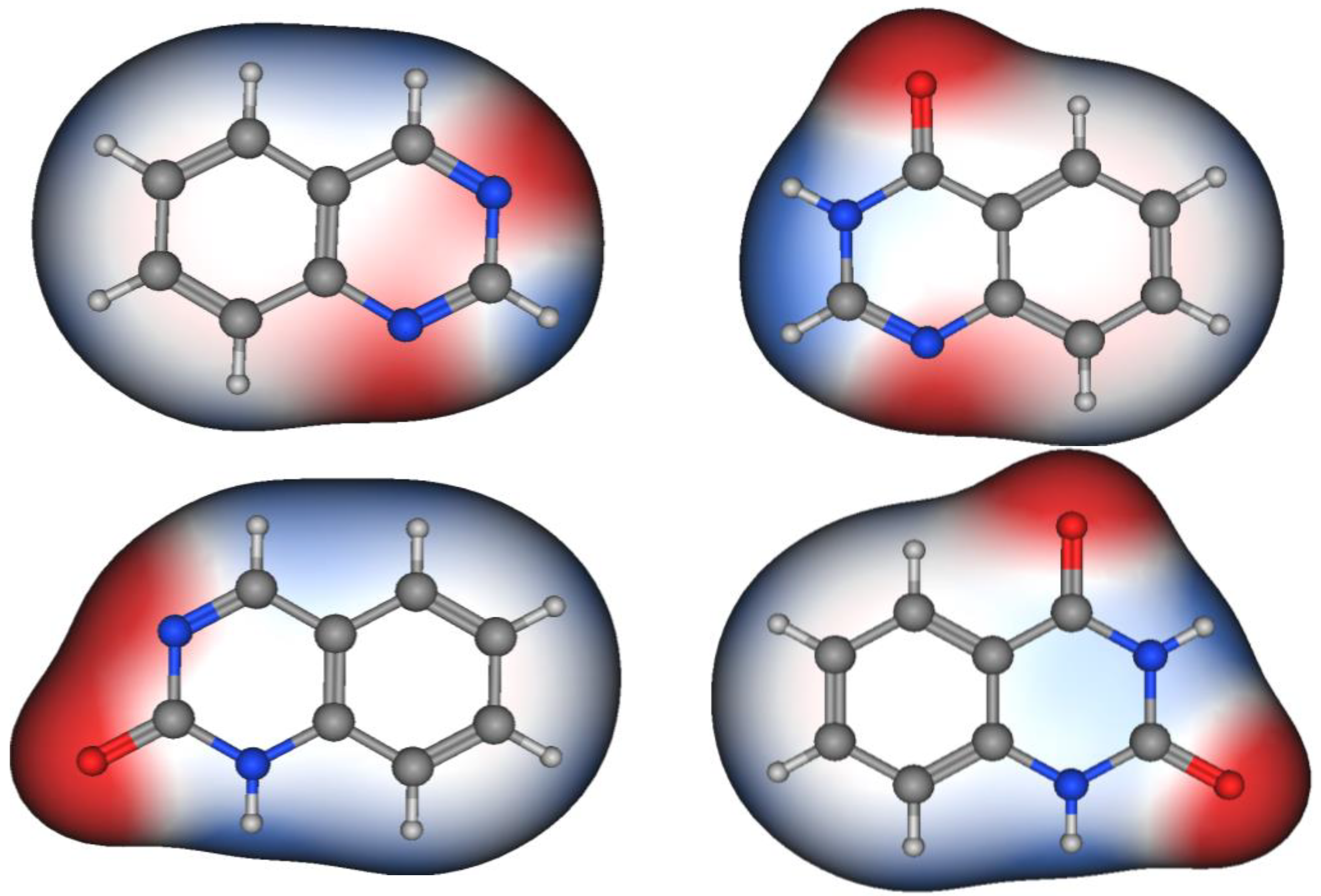
Figure 4. Electrostatic potential of quinazoline, 4-quinazolinone, 2-quinazolinone, and 2,4-quinazolinedione. Red color represents high polarity, white color represents neutral polarity, and blue color represents mild polarity.
Table 1. The physicochemical characters of quinazoline, 2-quinazolinone, 4-quinazolinone, and 2,4-quinazolinedione.
| Character | 2-Quinazolinone | 4-Quinazolinone | 2,4-Quinazolinedione |
|---|---|---|---|
| Molecular formula | C8H6N2 | C8H6N2O | C8H6N2O2 |
| Molecular weight | 130.15 g/mol | 146.15 g/mol | 162.15 g/mol |
| Number of heavy atoms | 10 | 11 | 12 |
| Number of aromatic heavy atoms | 10 | 10 | 10 |
| Fraction Csp3 | 0 | 0 | 0 |
| Number of rotatable bonds | 0 | 0 | 0 |
| Number of H-bond acceptors | 2 | 2 | 2 |
| Number of H-bond donors | 0 | 1 | 2 |
| Molar refractivity | 39.54 | 42.36 | 45.19 |
| Tropological polar surface area | 25.78 A2 | 45.75 A2 | 65.72 |
| Lipophilicity | 1.46 | 1.14 | 1.04 |
| Water solubility | Soluble | Soluble | Soluble |
| GI absorption | High | High | High |
| BBB permeation | Yes | Yes | No |
| Bioavailability score | 0.55 | 0.55 | 0.55 |
| Lipinski | Yes | Yes | Yes |
| Synthetic accessibility | Very easy | Very easy | Very easy |
3. Methods of Preparation of Quinazolines
- 1.
-
The Niemetowski method (Scheme 1) is based on the reaction of anthranilic acid and formamide under high temperature to yield 4-(3H)-quinazolinone [25][28].
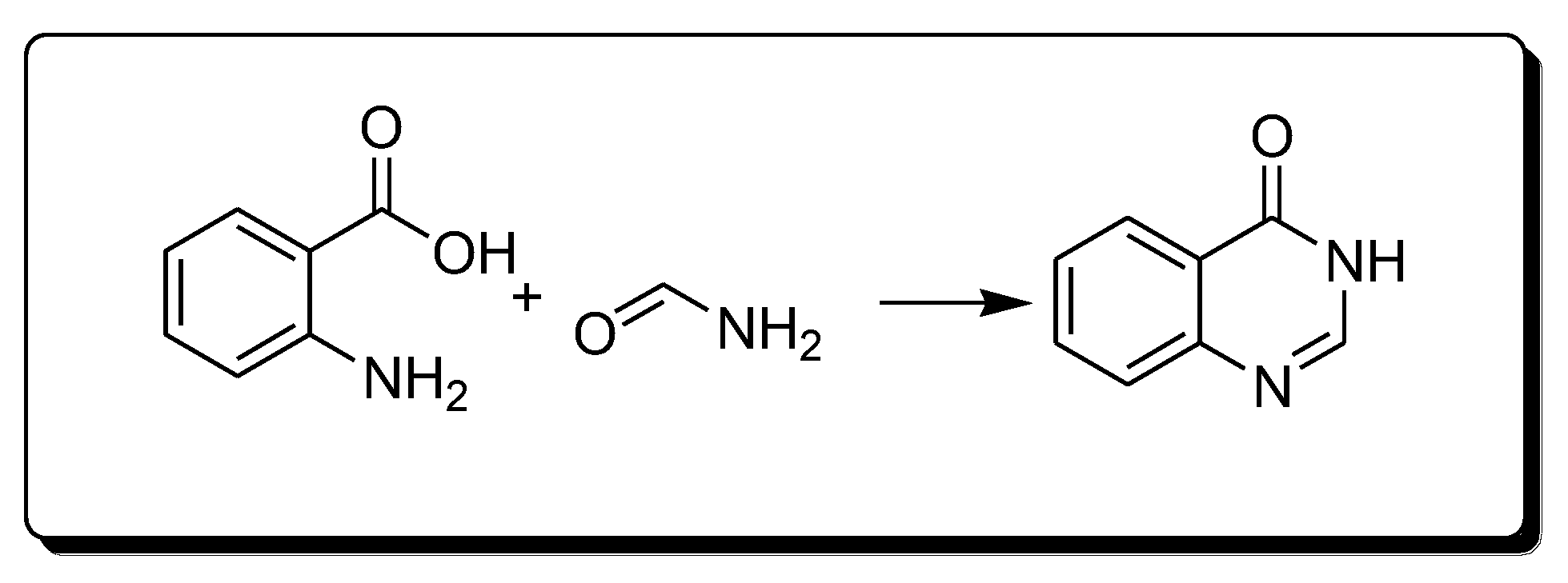 Scheme 1. Synthesis of 4-quinazolinone by Niemetowski method.
Scheme 1. Synthesis of 4-quinazolinone by Niemetowski method.
- 2.
-
Morgan’s method for the synthesis of quinazoline (Scheme 2) uses the reaction between 2-acetamidobenzoic acid and an amine in the presence of phosphorous trichloride to yield 2-methyl-3-phenylquinazolin-4(3H)-one [25][28]
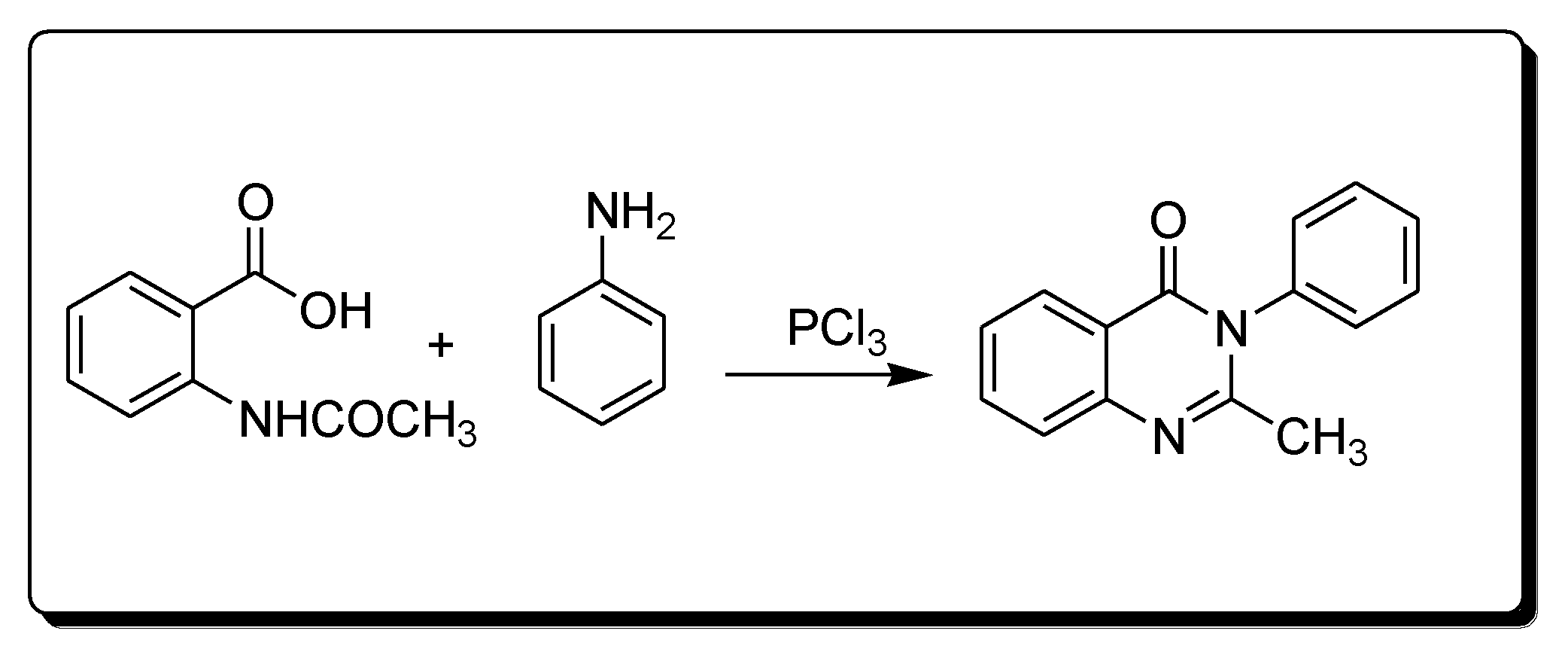 Scheme 2. Synthesis of 4-quinazolineone by Morgan’s method.
Scheme 2. Synthesis of 4-quinazolineone by Morgan’s method.
- 3.
-
The reaction between isatoic anhydride and an amine, followed by refluxing with ethyl orthoformate (Scheme 3) produces 4-(3H)-quinazolinone [25][29].
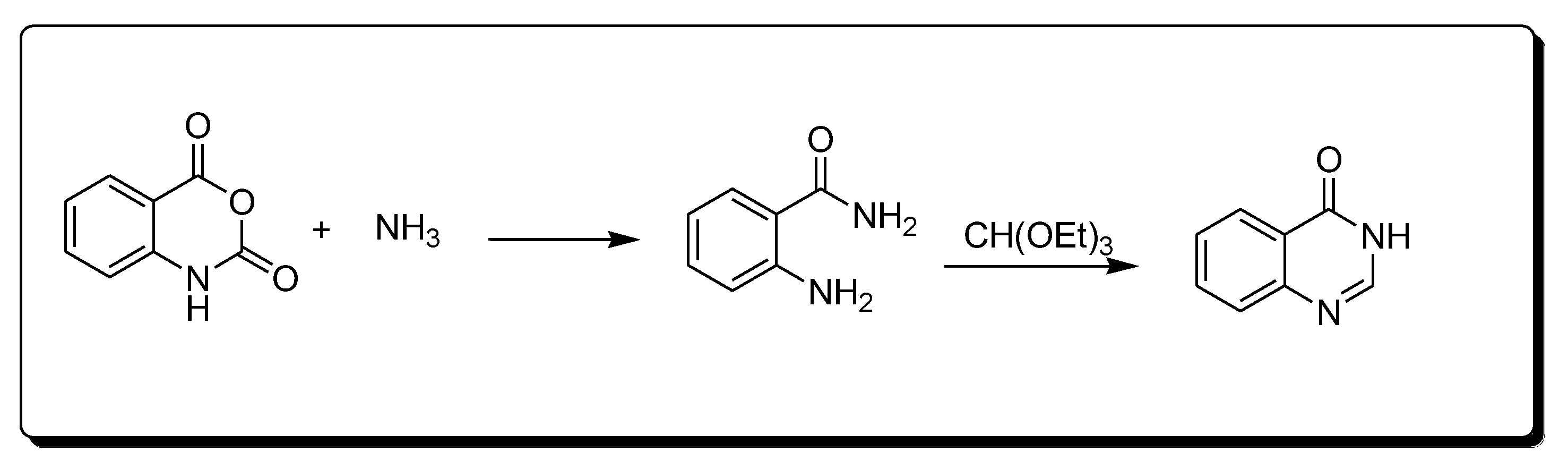 Scheme 3. Synthesis of 4-quinazolinone.
Scheme 3. Synthesis of 4-quinazolinone.
- 4.
-
The reaction of amines 2-methyl-4-nitro-bezoxazine-4-one derivatives produces 2-methyl-4-nitro-quinazolin-4-one derivatives (Scheme 4) [28].
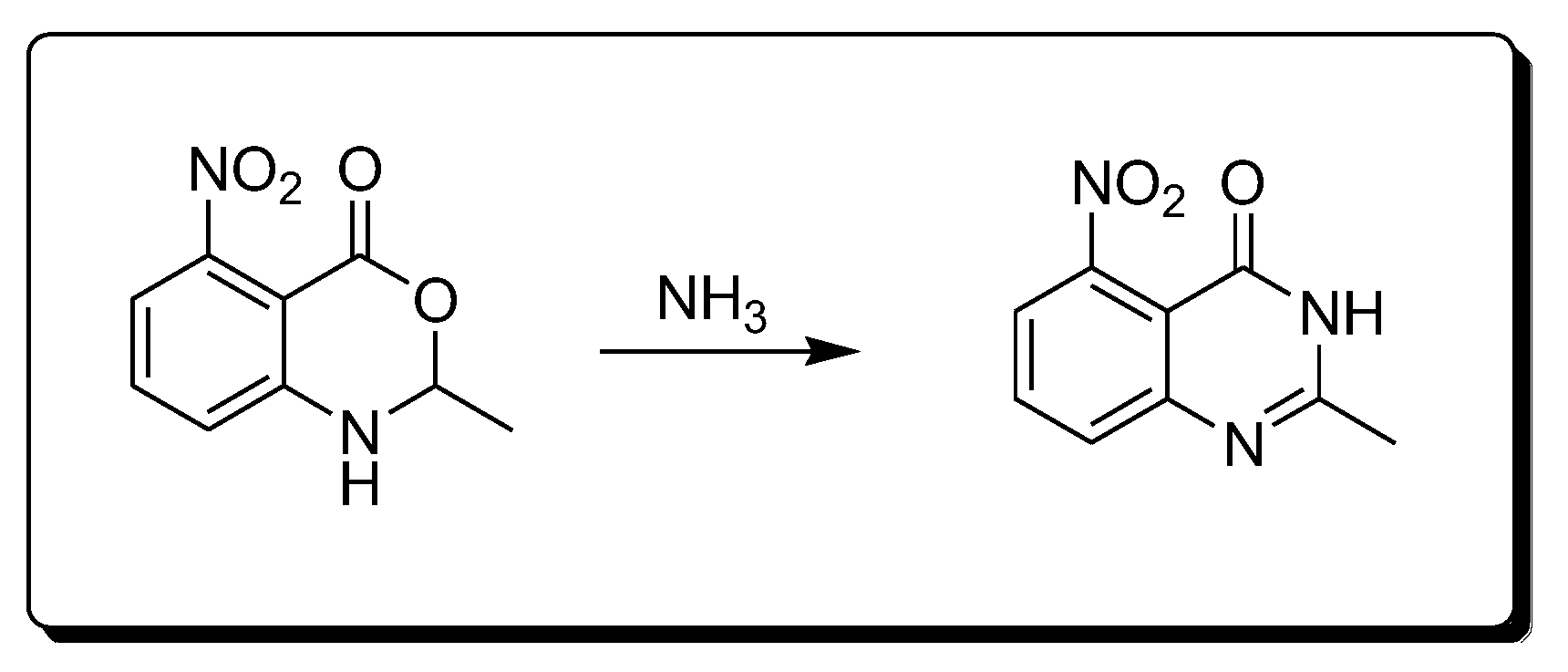 Scheme 4. Synthesis of 4-quinazolinone by amination reaction.
Scheme 4. Synthesis of 4-quinazolinone by amination reaction.
- 5.
-
Anthranilic acid and potassium cyanate react together (Scheme 5) to produce 2,4-quinazolinedione derivatives [29][30].
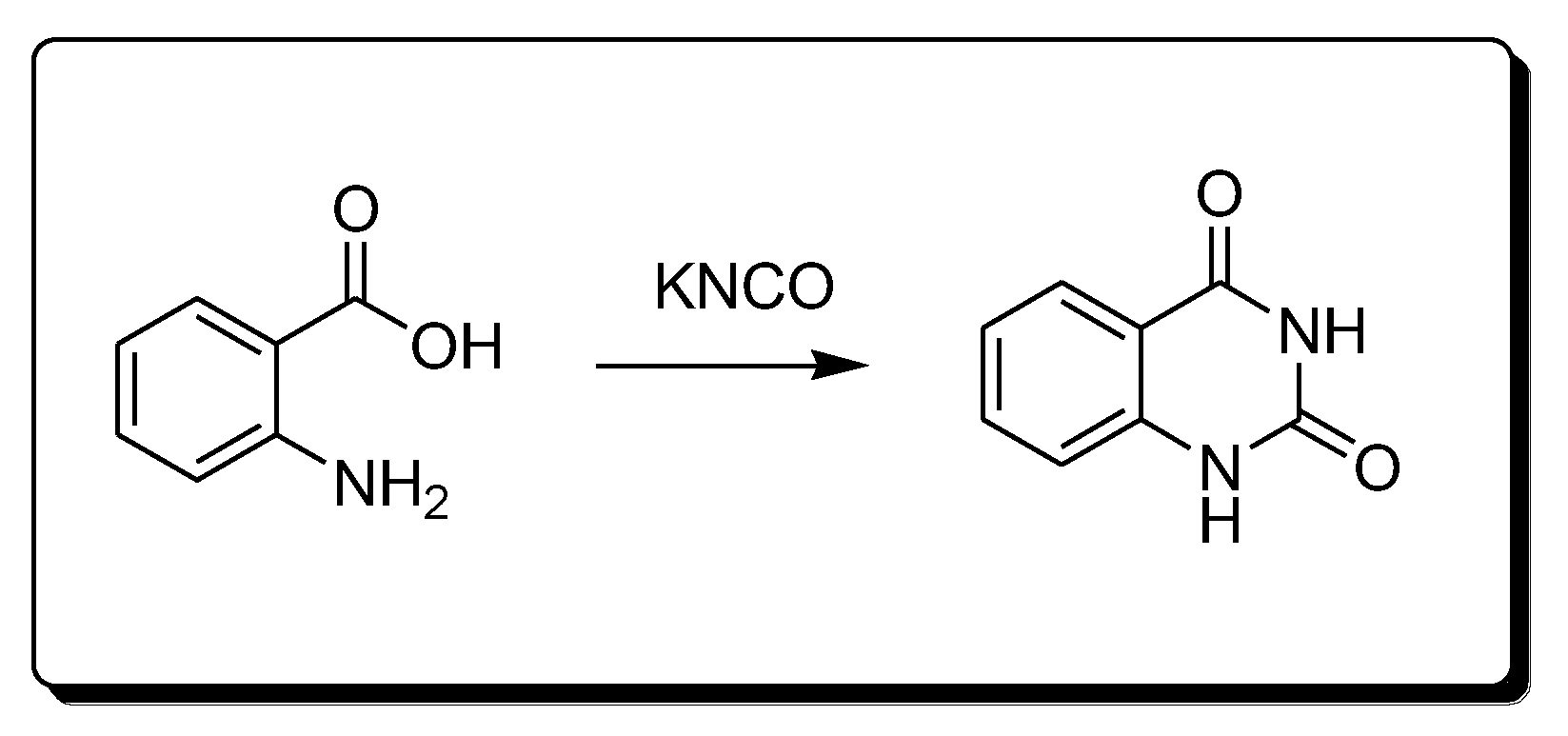 Scheme 5. Synthesis of 2,4-quinazolindione.
Scheme 5. Synthesis of 2,4-quinazolindione.
- 6.
-
The reaction of 2-aminobenzamide and styrene in the presence of Di-tertiary-butyl peroxide (DTBP) and P-toluene sulfonic acid (p-TsOH) produces 2-phenylquinazoline-4(3H)-one derivatives (Scheme 6) [29][30].
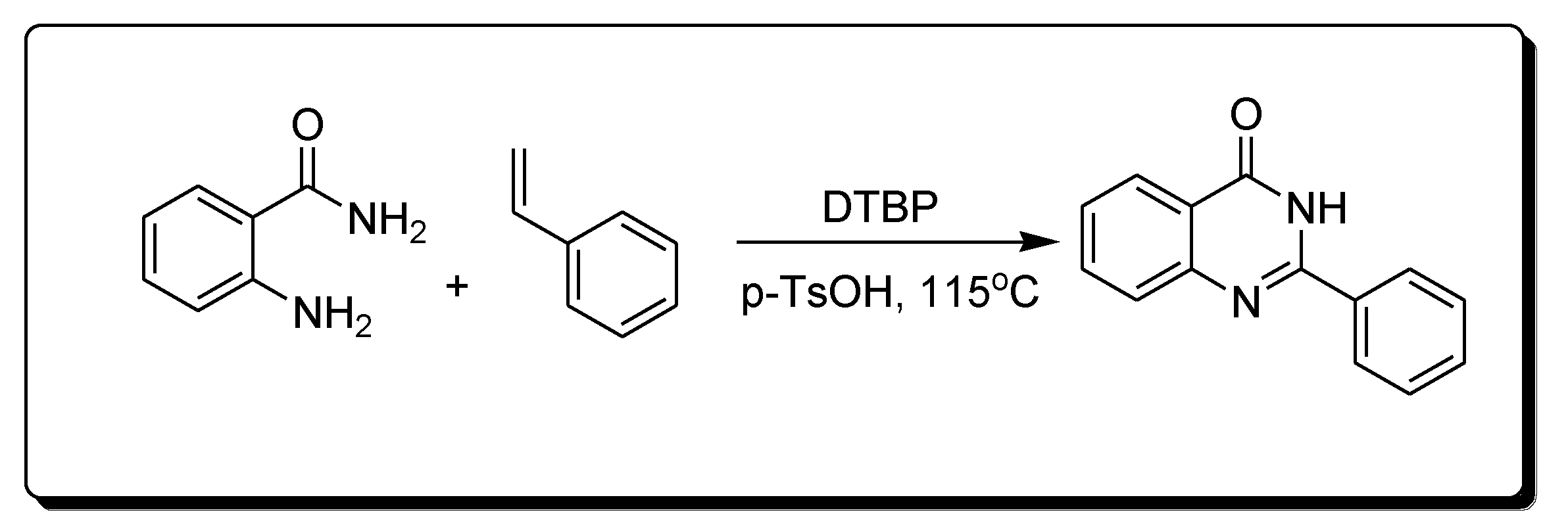 Scheme 6. Synthesis of 4-quinazolinone.
Scheme 6. Synthesis of 4-quinazolinone.
- 7.
-
Transition metals are catalyzed by the synthesis of quinazoline (Scheme 7) [30]. This method is based on the catalytic reduction in the nitro benzamide derivative using palladium chloride (PdCl2) and iron pentacarbonyl Fe(CO)5.
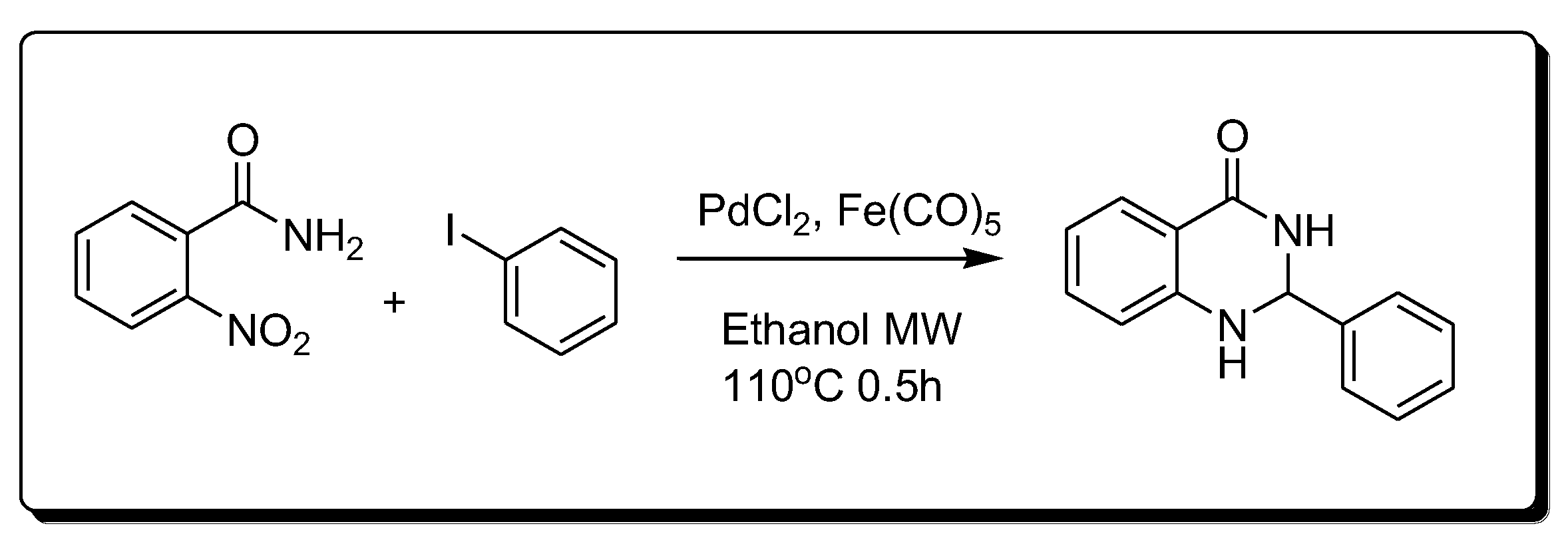 Scheme 7. Synthesis of 4-quinazolinone by transition-metal-catalyzed method.
Scheme 7. Synthesis of 4-quinazolinone by transition-metal-catalyzed method.
4. Analgesic Activity
Many quinazolines have been synthesized and evaluated for their analgesic and anti-inflammatory activities [31][32][33][34][35][36][37][38]. 2-Phenyl quinazolinone 4 (Figure 5) was synthesized by Alagarsamy et al. in 2002 [39]. It was biologically evaluated as an analgesic agent. The structure–activity relationship study explained that the highest activity was obtained by the compound with diethyl substitution, while aromatic and alicyclic amine substitution decreased analgesic activity. This activity was 58 ± 0.45% at 2 h and at 20 mg/kg compared to that of standard diclofenac sodium, which is 53 ± 0.35% at 2 h at 20 mg/kg. The modification of compound 4 to thiourea-substituted 2-methyl quinazolinone derivatives 5 produced more active compounds. The most active one was the compound which had the pyrrolidine ring at C-3 (Figure 5) [40]. The activity of this compound was 65 ± 0.79% at 2 h at 20 mg/kg. The activity of standard diclofenac during this experiment was 60 ± 0.54% at 1 h at 20 mg/kg. Further modification to thiourea-substituted 2-methylthio quinazolinone 6 yielded higher activity, i.e., 67 ± 1.18% at 2 h at 20 mg/kg [41]. Increasing lipophilicity at C-2 by placing the butyl group instead of the methyl group yielded a more active compound 7 with 73 ± 1.49% analgesic activity at 2 h at 20 mg/kg. Standard diclofenac produced 62 ± 1.49% analgesic activity at 2 h at 20 mg/kg. Placing the benzylamino group at C-2 produced active compound 8 with 55 ± 0.36% analgesic activity at 2 h at 20 mg/kg [42]. This activity was the same as standard diclofenac. Methylamino substituted 2-phenylquinazolinones 9 were synthesized and evaluated for analgesic activity [43]. They yielded 43 ± 0.51 to 61 ± 1.08% analgesic activity at 2 h at 20 mg/kg.
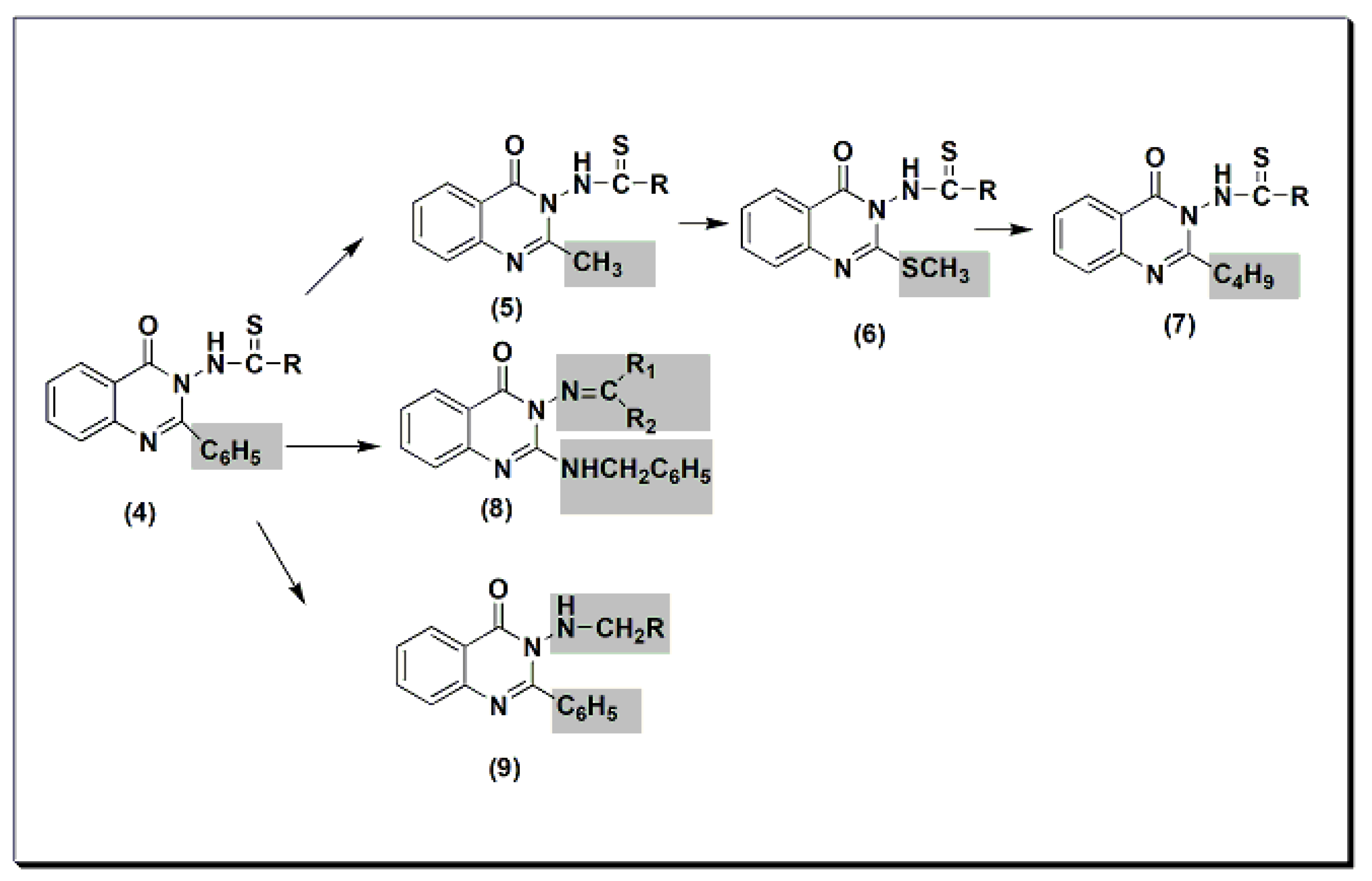
Figure 5. The analgesic quinazolines 4, 5, 6, 7, 8, and 9.
5. Anti-Inflammatory Activity
The previously discussed compounds (4–23) were evaluated for their anti-inflammatory activity. All these derivatives showed anti-inflammatory activity except the compound 20. It showed low anti-inflammatory activity when it was unsubstituted, while substitution with Cl at C-6 and CH3 at C-8 showed good activity.
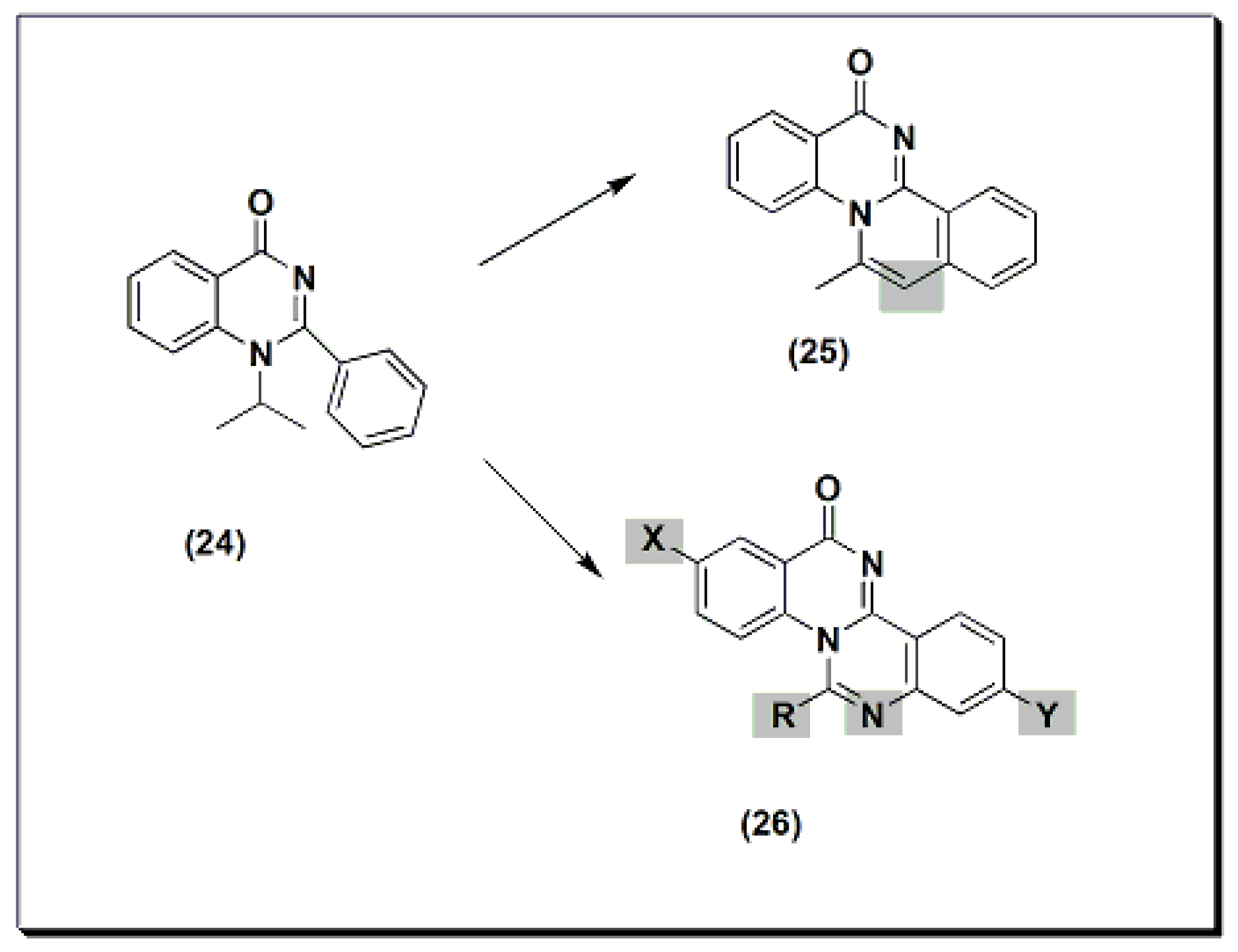
Two new derivatives of quinazolines, 25 and 26 (Figure 6), were designed, synthesized, and evaluated for their anti-inflammatory activity [44]. These derivatives were substituted isoquino-quinazolinone 25 and substituted quinazolino-quinazolinone 26. They were compared to the potent substituted 2-phenyl-quinazoline compound 24, but both were less active than compound 24.

Figure 6. The anti-inflammatory quinazolines 24, 25, and 26.
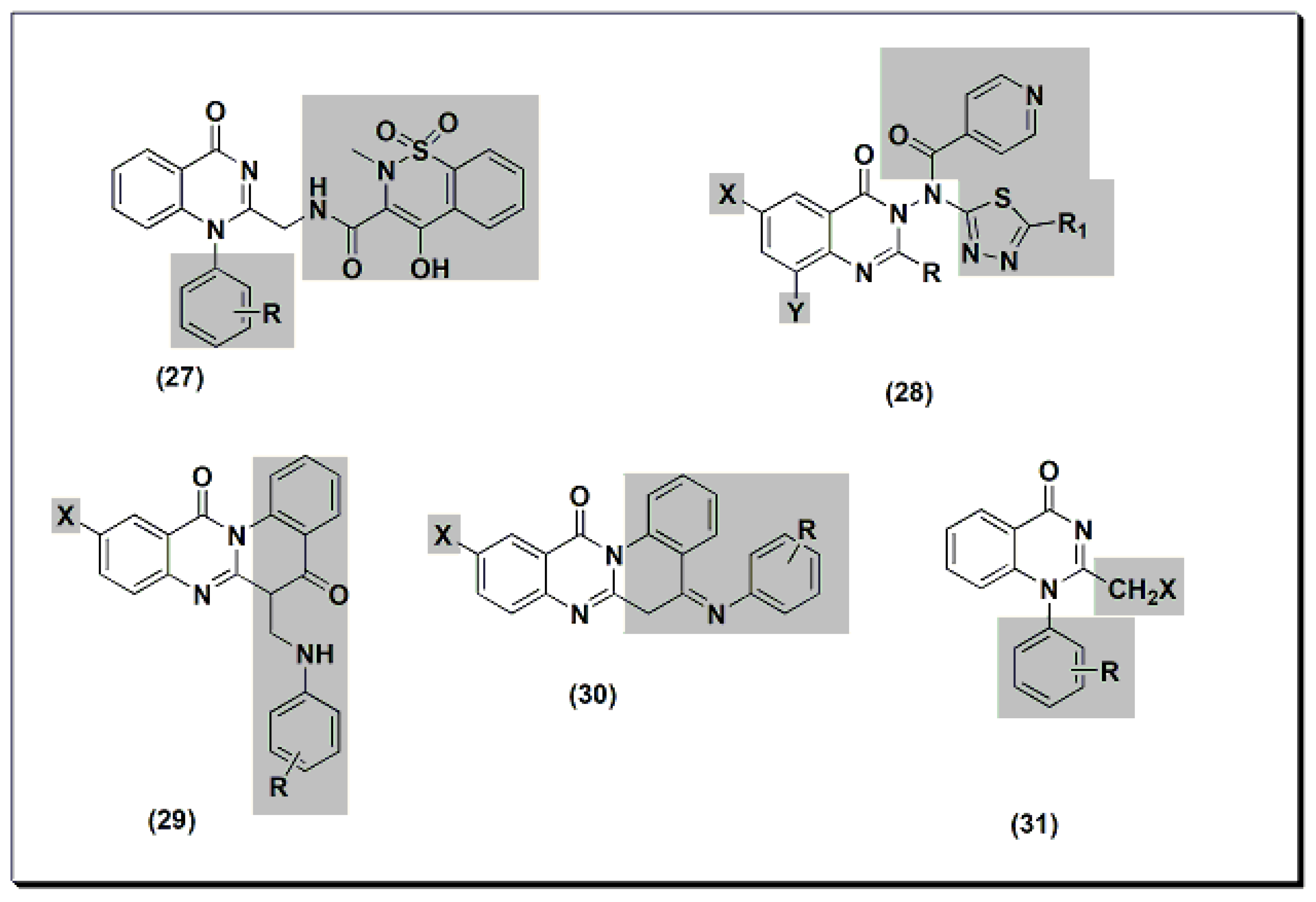
Other derivatives of compound 27 (Figure 7) were tested for their anti-inflammatory activity at 8 mg/kg using standard piroxicam at 4 mg/kg [45]. An SAR study showed that unsubstituted, 4-methyl, and 3-nitro derivatives of compound 27 yielded better activity than standard piroxicam. Using a 4 mg/kg dose yielded lower activity than piroxicam or diclofenac for the three derivatives. A structural modification was conducted by the introduction of the nicotinyl thiadiazole group at C-3 of 28 to improve the anti-inflammatory activity [46]. Nicotinyl-5-pyridyl thiadiazole of substituted 2-phenylquinazolinone yielded the highest activity, equivalent to standard ibuprofen. Substituted quino-quinazolinedione derivatives 29 and 30 showed an activity range of 12.7–58.2%. SAR studies showed that 10-iodosubstitution resulted in a significant increase in anti-inflammatory activity.

Figure 7. The anti-inflammatory quinazolines 27, 28, 29, 30, and 31.
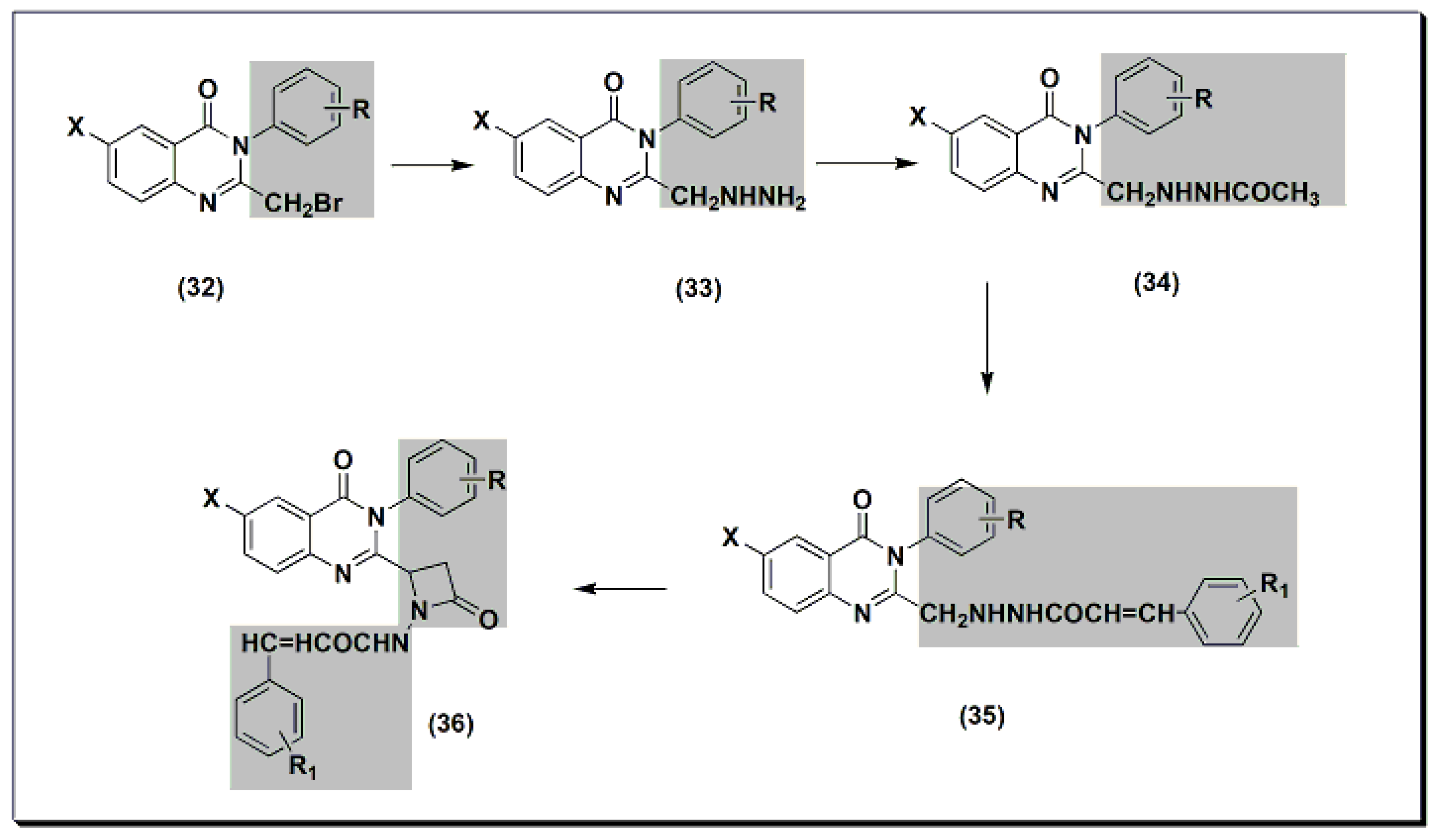
2,3,6-trisubstituted quinazolinone derivatives 32–36 (Figure 8) were designed, synthesized, and tested as anti-inflammatory agents [47]. These derivatives showed a variable activity range of 10.28–53.33%. Compounds with o-methoxyphenyl substituents at C-3 and p-dimethylaminophenyl at C-2 showed activity higher than standard phenylbutazone. In addition, these derivatives were tested for their ulcerogenic activity. The highly active anti-inflammatory derivatives were less ulcerogenic compared to standard phenylbutazone.

Figure 8. The anti-inflammatory quinazolines 32, 33, 34, 35, and 36.
6. Analgesic and Anti-Inflammatory Quinazolines Marketed Drugs
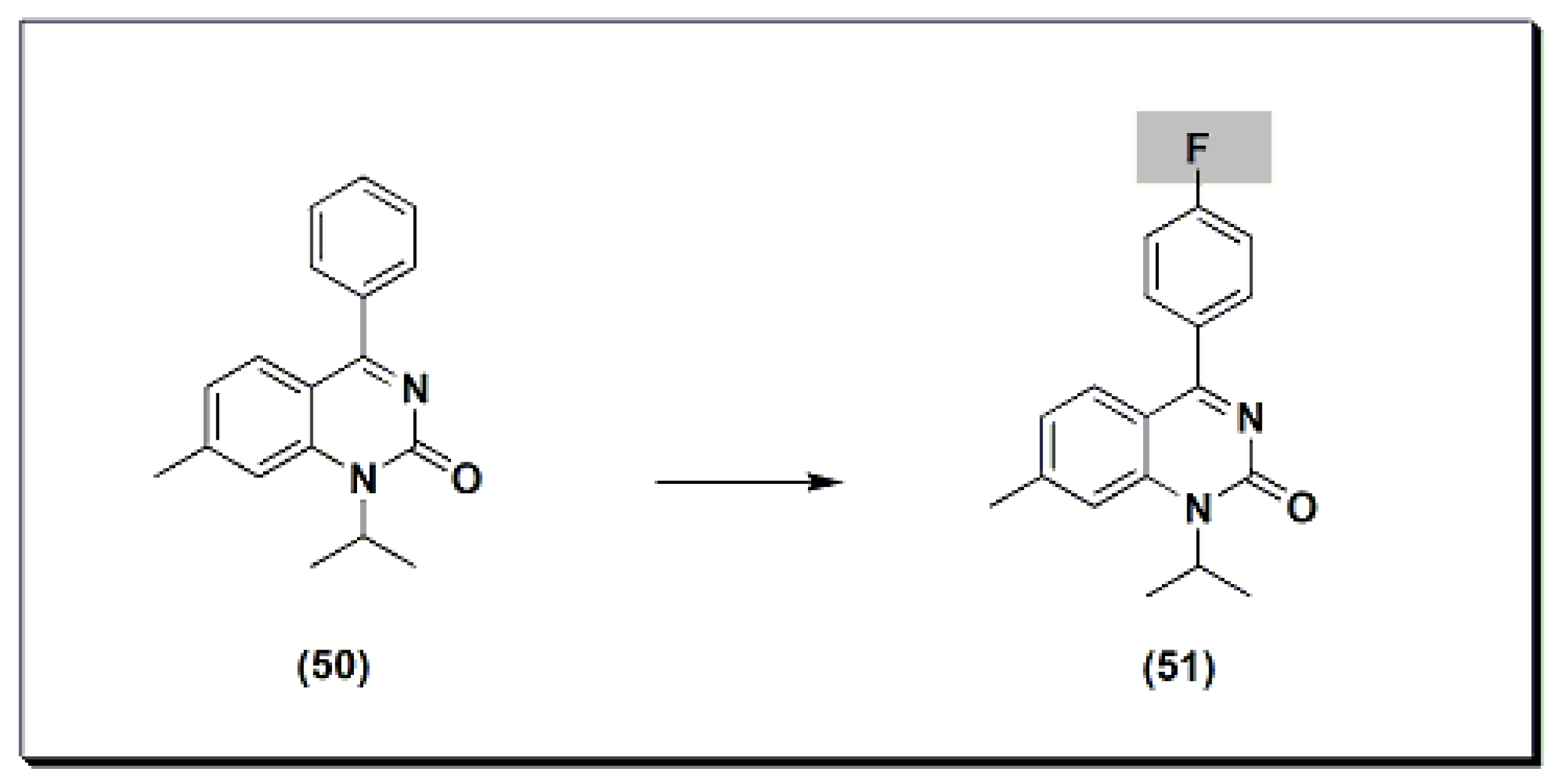
The marketed drug proquazone 50 (Figure 9) [48] is a quinazoline-based non-steroidal anti-inflammatory agent (NSAI). It is a 4-aryl-1-alkyl-quinazolinone derivative [48]. It is the first effective anti-inflammatory drug of a non-acidic nature. It is used in the treatment of rheumatoid arthritis and osteoarthritis. The dose of 300 to 900 mg/day is as effective as aspirin and diclofenac in the patients with osteoarthritis.

Figure 9. The molecular structure of proquazone and fluproquazone.
The marketed quinazoline anti-inflammatory agent NSC127213 (Figure 10) [48] is a derivative of tetrazolo quinazoline [48]. NSC127213 works by inhibiting the histamine-1 receptor (H1R) and the histamine-4 receptor (H4R) for the treatment and prevention of inflammatory, autoimmune, and allergic diseases. The molecular structure of these marketed drugs was based on previous studies on analgesic anti-inflammatory quinazolines.

Figure 10. The molecular structure of NSC127213.
7. Conclusions
As mentioned above, the quinazoline system has a wide range of biological activities, such as being analgesic, anti-inflammatory, anti-diabetic, anti-hyperlipidemic, anti-hypertensive, anti-bacterial, anti-fungal, anti-viral, anti-malarial, anti-convulsant, anti-cancer, anti-depressant, and anti-tubercular agents, as well as other activities. Therefore, it has great therapeutic potential for the treatment of different types of diseases and infections. The study of the structural–activity relationship (SAR) is the key for the improvement of efficient quinazoline therapeutic agents. Optimized quinazoline derivatives will be produced via an SAR-based study. Structural modifications can be performed by different techniques, such as the molecular hybridization or bioisosteric replacement techniques.
This entry is adapted from the peer-reviewed paper 10.3390/chemengineering6060094
References
- El-Zahabia, M.A.; Bamanie, H.F.; Ghareeb, S.; Alshaeri, K.H.; Alasmari, M.M.; Muostafa, M.; Al-Marzoki, Z.; Zayed, M.F. Design, Synthesis, Molecular Modeling andAnti-Hyperglycemic Evaluation of Quinazoline-Sulfonylurea Hybrids as Peroxisome Proliferator-Activated Receptor Gamma (PPAR) and Sulfonylurea Receptor (SUR) Agonists. Int. J. Mol. Sci. 2022, 23, 9605.
- Zayed, M.F.; Ibrahim, S.; Habib, E.E.; Hassan, M.H.; Ahmed, S.; Rateb, H.S. Design, synthesis, antimicrobial and anti-biofilm evaluation, and molecular docking of new substituted fluoroquinazolinones. J. Med. Chem. 2019, 15, 657–673.
- Zhang, J.; Liu, J.; Ma, Y.; Ren, D.; Cheng, P.; Zhao, J.; Zhang, F.; Yao, Y. One-pot synthesis and antifungal activity against plant pathogens of quinazolinone derivatives containing an amide moiety. Bioorg. Med. Chem. Lett. 2016, 26, 2273–2277.
- Zayed, M.F.; Rateb, H.; Ahmed, S.; Khaled, O.; Ibrahim, S. Quinazolinone-amino acid hybrids as Dual Inhibitors of EGFR Kinase and Tubulin Polymerization. Molecules 2018, 23, 1699.
- Santos-Ballardo, L.; García-Páez, F.; Picos-Corrales, L.A.; Ochoa-Terán, A.; Bastidas, P.; Calderón-Zamora, L.; Rendón-Maldonado, G.; Osuna-Martínez, U.; Sarmiento-Sánchez, J.I. Synthesis, biological evaluation and molecular docking of 3-substituted quinazoline-2,4(1H, 3H)-diones. J. Chem. Sci. 2020, 132, 100.
- Zayed, M.F.; Ahmed, S.; Ihmaid, S.; Ahmed, H.E.; Rateb, H.; Ibrahim, S. Design, Synthesis, Cytotoxic Evaluation and Molecular Docking of New Fluoroquinazolinones as Potent Anticancer Agents with Dual EGFR Kinase and Tubulin Polymerization Inhibitory Effects. Int. J. Mol. Sci. 2018, 19, 1731.
- Jain, R.K.; Kashaw, V. Design, synthesis and evaluation of novel 2,3-disubstituted-4-(3H) quinazolinone derivatives. Asian J. Pharm. Pharmacol. 2018, 4, 644–656.
- Alagarsamy, V.; Chitra, K.; Saravanan, G.; Solomon, V.R.; Sulthana, M.T.; Narendhar, B. An overview of quinazolines: Pharmacological significance and recent developments. Eur. J. Med. Chem. 2018, 151, 628–685.
- Zayed, M.F.; Ahmed, H.E.A.; Ihmaid, S.; El-Adl, K.; Asiri, A.; Omar, A.M. Modelling and Anticonvulsant Studies of New Quinazolines Showing Three Highly Active Compounds with Low Toxicity and High Affinity to the GABA-A Receptor. Molecules 2017, 22, 188.
- Hricoviniova, J.; Hricoviniova, Z.; Kozica, K. Antioxidant, Cytotoxic, Genotoxic, and DNA Protective Potential of 2,3-Substituted Quinazolinones: Structure—Activity Relationship Study. Int. J. Mol. Sci. 2021, 22, 610.
- Zayed, M.F.; Ahmed, H.E.A.; Ihmaid, S.K.; Omar, A.M.; Abdelrahim, A.S. Synthesis and screening of some new fluorinated quinazolinone–sulphonamide hybrids as anticancer agents. J. Taibah Univ. Sci. 2015, 10, 333–339.
- Sunil Kumar, A.; Kudva, J.; Lahtinen, M.; Peuronen, A.; Sadashiva, R.; Naral, D. Synthesis, characterization, crystal structures and biological screening of 4-amino quinazoline sulfonamide derivatives. J. Mol. Struct. 2019, 1190, 29–36.
- Zayed, M.F. New fluorinated quinazolinone derivatives as anticonvulsant agents. J. Taibah Univ. Sci. 2014, 9, 104–109.
- Ghorab, M.M.; Abdel-Kader, M.S.; Alqahtani, A.S.; Soliman, A.M. Synthesis of some quinazolinones inspired from the natural alkaloid L-norephedrine as EGFR inhibitors and radiosensitizers. J. Enzym. Inhib. Med. Chem. 2021, 36, 218–237.
- Zayed, M.F.; Hassan, M.H. Synthesis and biological evaluation studies of novel quinazolinone derivatives as antibacterial and anti-inflammatory agents. Saudi Pharm. J. 2014, 22, 157–162.
- Zayed, M.F.; Hassan, M.H. Design, Synthesis and Biological Evaluation Studies of Novel Quinazoline Derivatives as Cytotoxic Agents. Drug Res. 2013, 63, 210–215.
- Zayed, M.F.; Ahmed, E.A.; Omar, A.M.; Abdelrahim, A.S.; El-Adl, K. Design, synthesis, and biological evaluation studies of novel quinazolinone derivatives as anticonvulsant agents. Med. Chem. Res. 2013, 22, 5823–5831.
- Alam, M.J.; Alam, O.; Naim, M.J.; Alam, P. A review: Recent investigations on quinazoline scaffold. Int. J. Adv. Res. 2015, 3, 1656–1664.
- Connolly, D.J.; Cusack, D.; O’Sullivan, T.P.; Guiry, P.J. Synthesis of quinazolinones and quinazolines. Tetrahedron 2005, 61, 10153–10202.
- Meyer, J.F.; Wagner, E.C. The Niementowski reaction. The use of methyl anthranilate or isatoic anhydride with substituted amides or amidines in the formation of 3-substituted-4-keto-3,4-dihydroquinazolines. The course of the reaction. J. Org. Chem. 1943, 8, 239–252.
- Asif, M. Chemical Characteristics, Synthetic Methods, and Biological Potential of Quinazoline and Quinazolinone Derivatives. Int. J. Med. Chem. 2014, 2014, 395637.
- Hameed, A.; Al-Rashida, M.; Uroos, M.; Ali, S.A.; Arshia; Ishtiaq, M.; Khan, K.M. Quinazoline and quinazolinone as important medicinal scaffolds: A comparative patent review (2011–2016). Expert Opin. Ther. Pat. 2018, 28, 281–297.
- Auti, P.S.; George, G.; Paul, A.T. Recent advances in the pharmacological diversification of quinazoline/quinazolinone hybrids. RSC Adv. 2020, 10, 41353–41392.
- Gupta, T.; Rohilla, A.; Pathak, A.; Akhtar, M.J.; Haider, M.R.; Yar, M.S. Current perspectives on quinazolines with potent biological activities: A review. Synth. Commun. 2018, 48, 1099–1127.
- Bisht, A.S.; Negi, J.S.; Sharma, D.K. Chemistry and activity of quinazoline moiety: A systematic review study. Int. J. Pharm. Chem. Anal. 2020, 7, 61–65.
- Molecular Operating Environment (MOE). Chemical Computing Group. Available online: http://www.chemcomp.com (accessed on 10 May 2019).
- Swiss Institute of Bioinformatics (SwissADME). Available online: http://www.swissADME.ch (accessed on 10 March 2022).
- Wang, D.; Gao, F. Quinazoline derivatives: Synthesis and bioactivities. Chem. Cent. J. 2013, 7, 95.
- Agrawal, G.; Khan, N.; Dwivedi, S.; Patel, R.; Singh, G. Synthesis, Characterization and Anti-Microbial Evaluation of a Series of Quinazoline analogs. J. Adv. Sci. Res. 2021, 12, 123–130.
- Reddy, M.M.; Sivaramakrishna, A. Remarkably flexible quinazolinones—Synthesis and biological applications. J. Heterocycl. Chem. 2020, 57, 942–954.
- Kumar, A.; Sharma, S.; Bajaj, K.; Sharma, S.; Panwar, H.; Singh, T.; Srivastava, V.K. Some new 2,3,6-trisubstituted quinazolinones as potent anti-inflammatory, analgesic, and COX-II inhibitors. Bioorg. Med. Chem. 2003, 11, 5293–5299.
- Kumar, A.; Singh, R.C.; Kumar, B.S. Synthesis of 3--2--6-bromoquinazolin-4-ones as anti-inflammatory agent. Bioorg. Med. Chem. 2007, 15, 3089–3096.
- Pannerselvam, P.; Pradeepchandran, R.V.; Sridhar, S.K. Synthesis, characterization and biological activities of novel 2-methylquinazolin-4(3H)-ones. Indian J. Pharm. Sci. 2003, 65, 268–273.
- Gomtsyan, A.; Bayburt, E.K.; Schmidt, R.G.; Zheng, G.Z.; Perner, R.J.; Didomenico, S.; Koenig, J.R.; Turner, S.; Jinkerson, T.; Drizin, I.; et al. Novel transient receptor potential vanilloid 1 receptor antagonists for the treatment of pain: Structure activity relationships for ureas with quinoline, isoquinoline, quinazoline, phthalazine, quinoxaline, and cinnoline moieties. J. Med. Chem. 2005, 48, 744–752.
- Rakesh, K.P.; Manukumar, H.M.; Gowda, D.C. Schiff’s bases of quinazolinone derivatives: Synthesis and SAR studies of a novel series of potential anti-inflammatory and antioxidants. Bioorg. Med. Chem. Lett. 2015, 25, 1072–1077.
- Pu, Y.; Cao, D.; Xie, C.; Pei, H.; Li, D.; Tang, M.; Lijuan, C. Anti-arthritis effect of a novel quinazoline derivative through inhibiting production of TNF-α mediated by TNF-α converting enzyme in murine collagen-induced arthritis model. Biochem. Biophys. Res. Commun. 2015, 462, 288–293.
- Hu, J.; Zhang, Y.; Dong, L.; Wang, Z.; Chen, L.; Liang, D.; Shi, D.; Shan, X.; Liang, G. Design, synthesis, and biological evaluation of novel quinazoline derivatives as anti-inflammatory agents against lipopolysaccharide-induced acute lung injury in rats. Chem. Biol. Drug. Des. 2015, 85, 672–684.
- Alagarsamy, V.; Solomon, V.R.; Vanikavitha, G.; Paluchamy, V.; Chandran, M.R.; Sujin, A.A.; Thangathiruppathy, A.; Amuthalakshmi, S.; Revathi, R. Synthesis, analgesic, anti-inflammatory and anti-bacterial activities of some novel 2-phenyl-3-substituted quinazolin-4(3H)-ones. Biol. Pharm. Bull. 2002, 25, 1432–1435.
- Alagarsamy, V.; Muruganantham, G.; Venkateshaperumal, R. Synthesis, analgesic, anti-inflammatory and anti-bacterial activities of some novel 2-methyl-3-substituted quinazolin-4(3H)-ones. Biol. Pharm. Bull. 2003, 26, 1711–1714.
- Alagarsamy, V.; Rajesh, R.; Ramaseshu, M.; Vijaykumar, S.; Ramseshu, K.V.; Duraianandakumar, T. Synthesis, analgesic, anti-inflammatory and anti-bacterial activities of some novel 2-methylthio-3-substitutedquinazolin-4(3H)-one. Biol. Pharm. Bull. 2004, 27, 652–656.
- Alagarsamy, V.; Solomon, V.R.; Meena, R.; Ramseshu, K.V. Synthesis, analgesic, anti-inflammatory and anti-bacterial activities of some novel 2-butyl-3-substituted quinazolin-4(3H)-ones. Biol. Pharm. Bull. 2005, 28, 1091–1094.
- Alagarsamy, V.; Muthukumar, V.; Pavalarani, N.; Vasanthanathan, P.; Revathi, R. Synthesis, analgesic, and anti-inflammatory activities of some novel 2,3-disubstituted quinazolin-4(3H)-ones. Biol. Pharm. Bull. 2003, 26, 557–559.
- Alagarsamy, V.; Revathi, S.; Kalaiselvi, R. Analgesic, anti-inflammatory and antibacterial activity of some novel 2-phenyl-3-(substitutedmethylamino) quinazolin- 4(3H)-ones. Indian J. Pharm. Sci. 2003, 65, 534–537.
- Ozaki, K.I.; Yamada, Y.; Oine, T. Studies on 4(1H)-quinazolinones. IV: Convenient syntheses of 12-methyl-6H-isoquinoquinazolin-6-one and 6-methyl-13Hquinazolinoquinazolin-13-one. Chem. Pharm. Bull. 1984, 32, 2160–2164.
- Bothara, K.G.; Kadam, S.S.; Shivram, V.S. Synthesis, and pharmacological screening of novel anti-inflammatory agents. Indian Drugs 1998, 35, 372–376.
- Sarma, G.V.S.R.; Thomas, J.; Murugan, V.; Elango, K. Nicotinyl incorporated quinazolinonyl thiadiazoles as possible NSAIDs. Indian J. Heterocycl. Chem. 1999, 9, 151–152.
- Rani, P.; Archana; Srivastava, V.K.; Kumar, A. Synthesis and anti-inflammatory activity of some new 2,3-disubstituted 6-monosubstitutedquinazolin-4(3H)-ones. Indian J. Chem. 2002, 41B, 2642–2646.
- Drug Information Portal. Available online: https://druginfo.nlm.nih.gov/drugportal/ (accessed on 25 September 2022).
This entry is offline, you can click here to edit this entry!
