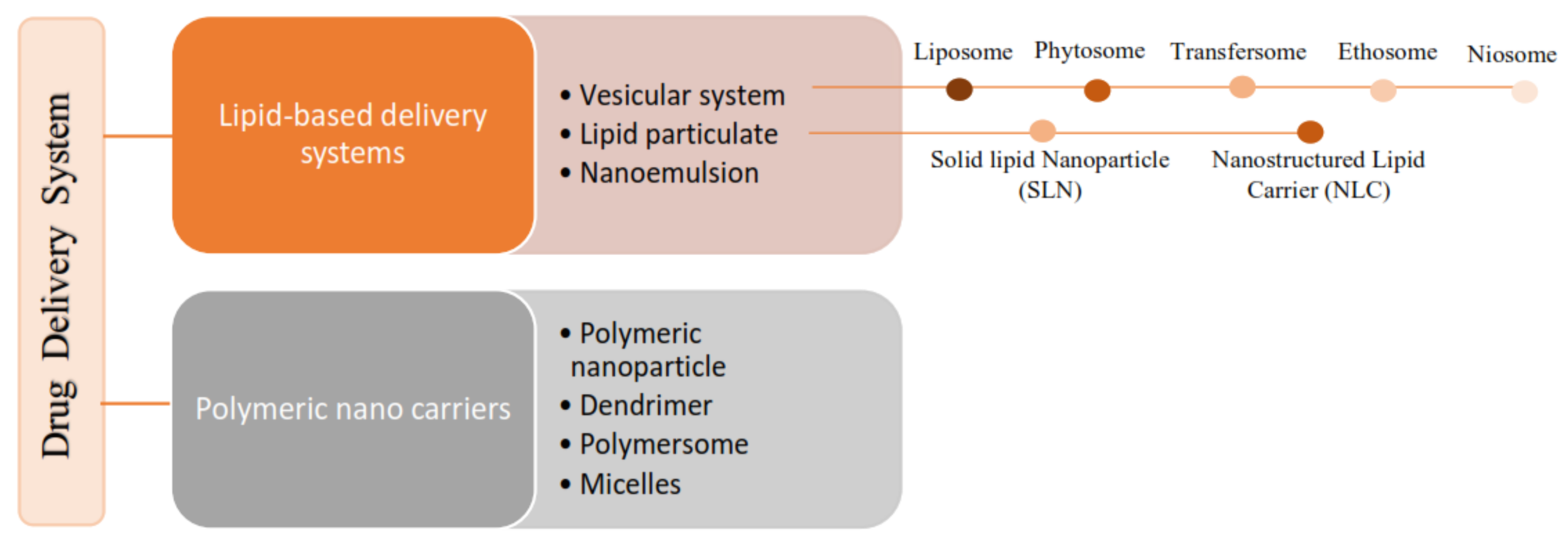
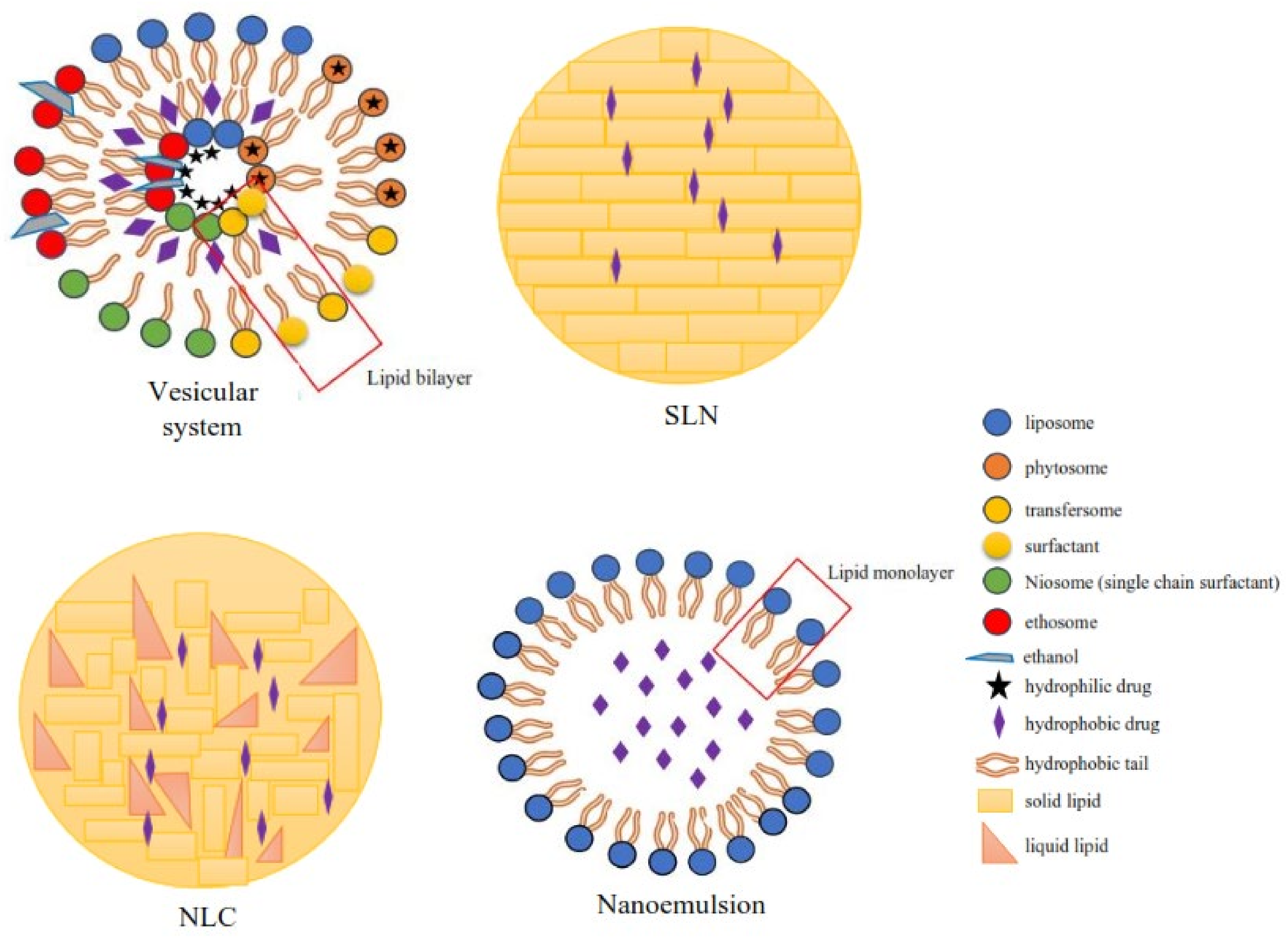
According to the literature, 70% of the active ingredients obtained from plants are hydrophobic. New technology has been used as a strategy to increase the bioavailability/bioactivity of phytochemical compounds. In order to develop new nanotechnology-based therapies, the ability to design suitable formulations for drug delivery is of the utmost importance. Phytochemical delivery is essential for effective disease prevention and treatment. These delivery systems include lipid-based delivery systems and polymer-based delivery systems, which have the potential to increase the bioactivity of phytochemical compounds.
- phytochemical
- herbal medicine
- drug delivery
1. Introduction
2. Nanotechnology-Based Drug Delivery System for Phytochemical Compounds
2.1. Phytosomes Increasing the Activity of Phytochemical Compounds
2.2. Polymeric Nanoparticle to Increase the Activity of Phytochemical Compounds
This entry is adapted from the peer-reviewed paper 10.3390/nano12224073
References
- Ijaz, S.; Akhtar, N.; Khan, M.S.; Hameed, A.; Irfan, M.; Arshad, M.A.; Ali, S.; Asrar, M. Plant Derived Anticancer Agents: A Green Approach towards Skin Cancers. Biomed. Pharmacother. 2018, 103, 1643–1651.
- Enrico, C. Nanotechnology-Based Drug Delivery of Natural Compounds and Phytochemicals for the Treatment of Cancer and Other Diseases. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; Volume 62.
- Martins-Gomes, C.; Souto, E.B.; Silva, A.M. Nanophytosomes: A Novel Approach for the Delivery of Herbal Drugs. In Systems of Nanovesicular Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2022; pp. 239–257.
- Hussein, R.A.; El-Anssary, A.A. Plants Secondary Metabolites: The Key Drivers of the Pharmacological Actions of Medicinal Plants. In Herbal Medicine; IntechOpen: London, UK, 2019.
- Mendoza, N.; Silva, E.M.E. Introduction to Phytochemicals: Secondary Metabolites from Plants with Active Principles for Pharmacological Importance. In Phytochemicals-Source of Antioxidants and Role in Disease Prevention; InTech: London, UK, 2018.
- Ahmed, H.M.; Nabavi, S.; Behzad, S. Herbal Drugs and Natural Products in the Light of Nanotechnology and Nanomedicine for Developing Drug Formulations. Mini-Rev. Med. Chem. 2020, 21, 302–313.
- Ahmed, H.M. Ethnopharmacobotanical Study on the Medicinal Plants Used by Herbalists in Sulaymaniyah Province, Kurdistan, Iraq. J. Ethnobiol. Ethnomed. 2016, 12, 1–17.
- Wang, S.; Su, R.; Nie, S.; Sun, M.; Zhang, J.; Wu, D.; Moustaid-Moussa, N. Application of Nanotechnology in Improving Bioavailability and Bioactivity of Diet-Derived Phytochemicals. J. Nutr. Biochem. 2014, 25, 363–376.
- Gunasekaran, T.; Haile, T.; Nigusse, T.; Dhanaraju, M.D. Nanotechnology: An Effective Tool for Enhancing Bioavailability and Bioactivity of Phytomedicine. Asian Pac. J. Trop Biomed. 2014, 4, S1–S7.
- Saraf, S.A. Applications of Novel Drug Delivery System for Herbal Formulations. Fitoterapia 2010, 81, 680–689.
- Singh, V.K.; Arora, D.; Ansari, M.I.; Sharma, P.K. Phytochemicals Based Chemopreventive and Chemotherapeutic Strategies and Modern Technologies to Overcome Limitations for Better Clinical Applications. Phytother. Res. 2019, 33, 3064–3089.
- Zhou, Y.X.; Zhang, H.; Peng, C. Puerarin: A Review of Pharmacological Effects. Phytother. Res. 2014, 28, 961–975.
- Quan, D.Q.; Xu, G.X.; Wu, X.G. Studies on Preparation and Absolute Bioavailability of a Self-Emulsifying System Containing Puerarin. Chem. Pharm. Bull 2007, 55, 800–803.
- Li, H.; Dong, L.; Liu, Y.; Wang, G.; Wang, G.; Qiao, Y. Biopharmaceutics Classification of Puerarin and Comparison of Perfusion Approaches in Rats. Int. J. Pharm. 2014, 466, 133–138.
- Luo, C.F.; Yuan, M.; Chen, M.S.; Liu, S.M.; Zhu, L.; Huang, B.Y.; Liu, X.W.; Xiong, W. Pharmacokinetics, Tissue Distribution and Relative Bioavailability of Puerarin Solid Lipid Nanoparticles Following Oral Administration. Int. J. Pharm. 2011, 410, 138–144.
- Luo, C.F.; Hou, N.; Tian, J.; Yuan, M.; Liu, S.M.; Xiong, L.G.; Luo, J.D.; Chen, M.S. Metabolic Profile of Puerarin in Rats after Intragastric Administration of Puerarin Solid Lipid Nanoparticles. Int. J. Nanomed. 2013, 8, 933–940.
- Bonifácio, B.V.; da Silva, P.B.; Aparecido dos Santos Ramos, M.; Maria Silveira Negri, K.; Maria Bauab, T.; Chorilli, M. Nanotechnology-Based Drug Delivery Systems and Herbal Medicines: A Review. Int. J. Nanomed. 2013, 9, 1–15.
- Ambwani, S.; Tandon, R.; Ambwani, T.K.; Malik, Y.S. Current Knowledge on Nanodelivery Systems and their beneficial Applications in Enhancing the Efficacy of Herbal Drugs. J. Exp. Biol. Agric. Sci. 2018, 6, 87–107.
- Fangueiro, J.F.; Souto, E.B.; Silva, A.M. Encapsulation of Nutraceuticals in Novel Delivery Systems. In Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2016.
- Souto, E.B.; Dias-Ferreira, J.; Oliveira, J.; Sanchez-Lopez, E.; Lopez-Machado, A.; Espina, M.; Garcia, M.L.; Souto, S.B.; Martins-Gomes, C.; Silva, A.M. Trends in Atopic Dermatitis—From Standard Pharmacotherapy to Novel Drug Delivery Systems. Int. J. Mol. Sci. 2019, 20, 5659.
- Paroha, S.; Dewangan, R.P.; Sahoo, P.K. Pharmaceutical Technology for Improving the Bioavailability of Natural Products. In Sustainable Agriculture Reviews 43; Springer: Berlin/Heidelberg, Germany, 2020.
- Sandhiya, V.; Ubaidulla, U. A Review on Herbal Drug Loaded into Pharmaceutical Carrier Techniques and Its Evaluation Process. Futur. J. Pharm. Sci. 2020, 6, 1–16.
- Mukherjee, P.K.; Harwansh, R.K.; Bhattacharyya, S. Bioavailability of Herbal Products: Approach Toward Improved Pharmacokinetics. In Evidence-Based Validation of Herbal Medicine; Elsevier: Amsterdam, The Netherlands, 2015.
- Tapadiya, G.G.; Kale, M.A.; Saboo, S.S. Impact of Nanotechnology on Global Trade of Herbal Drugs: An Overview. Int. J. Green Pharm. 2017, 11, S371–S376.
- Jain, S.; Jain, V.; Mahajan, S.C. Lipid Based Vesicular Drug Delivery Systems. Adv. Pharm. 2014, 2014, 574673.
- Sakellari, G.I.; Zafeiri, I.; Batchelor, H.; Spyropoulos, F. Formulation Design, Production and Characterisation of Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) for the Encapsulation of a Model Hydrophobic Active. Food Hydrocoll. Health 2021, 1, 100024.
- Kumar, M.; Bishnoi, R.S.; Shukla, A.K.; Jain, C.P. Techniques for Formulation of Nanoemulsion Drug Delivery System: A Review. Prev. Nutr. Food Sci. 2019, 24, 225–234.
- Németh, Z.; Pallagi, E.; Dobó, D.G.; Kozma, G.; Kónya, Z.; Csóka, I. An Updated Risk Assessment as Part of the QbD-Based Liposome Design and Development. Pharmaceutics 2021, 13, 1071.
- Liu, Y.; Castro Bravo, K.M.; Liu, J. Targeted Liposomal Drug Delivery: A Nanoscience and Biophysical Perspective. Nanoscale Horiz. 2021, 6, 78–94.
- Chavda, V.P.; Vihol, D.; Mehta, B.; Shah, D.; Patel, M.; Vora, L.K.; Pereira-Silva, M.; Paiva-Santos, A.C. Phytochemical-Loaded Liposomes for Anticancer Therapy: An Updated Review. Nanomedicine 2022, 17, 547–568.
- Kyriakoudi, A.; Spanidi, E.; Mourtzinos, I.; Gardikis, K. Innovative Delivery Systems Loaded with Plant Bioactive Ingredients: Formulation Approaches and Applications. Plants 2021, 10, 1238.
- Su, W.; Liang, Y.; Meng, Z.; Chen, X.; Lu, M.; Han, X.; Deng, X.; Zhang, Q.; Zhu, H.; Fu, T. Inhalation of Tetrandrine-Hydroxypropyl-β-Cyclodextrin Inclusion Complexes for Pulmonary Fibrosis Treatment. Mol. Pharm. 2020, 17, 1596–1607.
- Zhang, L.; Yang, S.; Wong, L.R.; Xie, H.; Ho, P.C.-L. In Vitro and in Vivo Comparison of Curcumin-Encapsulated Chitosan-Coated Poly (Lactic- Co-Glycolic Acid) Nanoparticles and Curcumin/Hydroxypropyl-β-Cyclodextrin Inclusion Complexes Administered Intranasally as Therapeutic Strategies for Alzheimer’s Disease. Mol. Pharm. 2020, 17, 4256–4269.
- Blasi, P.; Giovagnoli, S.; Schoubben, A.; Ricci, M.; Rossi, C. Solid Lipid Nanoparticles for Targeted Brain Drug Delivery. Adv. Drug Deliv. Rev. 2007, 59, 454–477.
- Song, J.-W.; Liu, Y.-S.; Guo, Y.-R.; Zhong, W.-X.; Guo, Y.-P.; Guo, L. Nano–Liposomes Double Loaded with Curcumin and Tetrandrine: Preparation, Characterization, Hepatotoxicity and Anti–Tumor Effects. Int. J. Mol. Sci. 2022, 23, 6858.
- Souto, E.B.; Fernandes, A.R.; Martins-Gomes, C.; Coutinho, T.E.; Durazzo, A.; Lucarini, M.; Souto, S.B.; Silva, A.M.; Santini, A. Nanomaterials for Skin Delivery of Cosmeceuticals and Pharmaceuticals. Appl. Sci. 2020, 10, 1594.
- Li, A.; Tyson, J.; Patel, S.; Patel, M.; Katakam, S.; Mao, X.; He, W. Emerging Nanotechnology for Treatment of Alzheimer’s and Parkinson’s Disease. Front. Bioeng. Biotechnol. 2021, 9, 322.
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.v.; Peppas, N.A. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173.
- Siegal, T. Which Drug or Drug Delivery System Can Change Clinical Practice for Brain Tumor Therapy? Neuro Oncol. 2013, 15, 656–669.
- Preethi, R.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Green Nanomaterials and Nanotechnology for the Food Industry. In Green Functionalized Nanomaterials for Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 215–256.
- Jie, M.; Cheung, W.M.; Yu, V.; Zhou, Y.; Tong, P.H.; Ho, J.W.S. Anti-Proliferative Activities of Sinigrin on Carcinogen-Induced Hepatotoxicity in Rats. PLoS ONE 2014, 9, e110145.
- Mazumder, A.; Dwivedi, A.; du Preez, J.L.; du Plessis, J. In Vitro Wound Healing and Cytotoxic Effects of Sinigrin-Phytosome Complex. Int. J. Pharm. 2016, 498, 283–293.
- Kumar, S.; Baldi, A.; Sharma, D.K. In Vitro Antioxidant Assay Guided Ex Vivo Investigation of Cytotoxic Effect of Phytosomes Assimilating Taxifolin Rich Fraction of Cedrus Deodara Bark Extract on Human Breast Cancer Cell Lines (MCF7). J. Drug Deliv. Sci. Technol. 2021, 63, 102486.
- Weidmann, A.E. Dihydroquercetin: More than Just an Impurity? Eur. J. Pharmacol. 2012, 684, 19–26.
- Luo, H.; Jiang, B.H.; King, S.M.; Chen, Y.C. Inhibition of Cell Growth and VEGF Expression in Ovarian Cancer Cells by Flavonoids. Nutr. Cancer 2008, 60, 800–809.
- Hasibi, F.; Nasirpour, A.; Varshosaz, J.; García-Manrique, P.; Blanco-López, M.C.; Gutiérrez, G.; Matos, M. Formulation and Characterization of Taxifolin-Loaded Lipid Nanovesicles (Liposomes, Niosomes, and Transfersomes) for Beverage Fortification. Eur. J. Lipid Sci. Technol. 2020, 122.
- Mondal, R.; Bobde, Y.; Ghosh, B.; Giri, T.K. Development and Characterization of a Phospholipid Complex for Effective Delivery of Capsaicin. Indian J. Pharm. Sci. 2019, 81, 1011–1019.
- Chi, C.; Zhang, C.; Liu, Y.; Nie, H.; Zhou, J.; Ding, Y. Phytosome-Nanosuspensions for Silybin-Phospholipid Complex with Increased Bioavailability and Hepatoprotection Efficacy. Eur. J. Pharm. Sci. 2020, 144, 105212.
- Theodosiou, E.; Purchartová, K.; Stamatis, H.; Kolisis, F.; Křen, V. Bioavailability of Silymarin Flavonolignans: Drug Formulations and Biotransformation. Phytochem. Rev. 2014, 13, 1–18.
- Shukla, S.; Gupta, S. Apigenin: A Promising Molecule for Cancer Prevention. Pharm. Res. 2010, 27, 962–978.
- Rithidech, K.N.; Tungjai, M.; Reungpatthanaphong, P.; Honikel, L.; Simon, S.R. Attenuation of Oxidative Damage and Inflammatory Responses by Apigenin given to Mice after Irradiation. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2012, 749, 29–38.
- Shibata, C.; Ohno, M.; Otsuka, M.; Kishikawa, T.; Goto, K.; Muroyama, R.; Kato, N.; Yoshikawa, T.; Takata, A.; Koike, K. The Flavonoid Apigenin Inhibits Hepatitis C Virus Replication by Decreasing Mature MicroRNA122 Levels. Virology 2014, 462–463, 42–48.
- Choi, J.S.; Nurul Islam, M.; Yousof Ali, M.; Kim, E.J.; Kim, Y.M.; Jung, H.A. Effects of C-Glycosylation on Anti-Diabetic, Anti-Alzheimer’s Disease and Anti-Inflammatory Potential of Apigenin. Food Chem. Toxicol. 2014, 64, 27–33.
- Zhang, J.; Liu, D.; Huang, Y.; Gao, Y.; Qian, S. Biopharmaceutics Classification and Intestinal Absorption Study of Apigenin. Int. J. Pharm. 2012, 436, 311–317.
- Chen, Z.; Kong, S.; Song, F.; Li, L.; Jiang, H. Pharmacokinetic Study of Luteolin, Apigenin, Chrysoeriol and Diosmetin after Oral Administration of Flos Chrysanthemi Extract in Rats. Fitoterapia 2012, 83, 1616–1622.
- Telange, D.R.; Patil, A.T.; Pethe, A.M.; Fegade, H.; Anand, S.; Dave, V.S. Formulation and Characterization of an Apigenin-Phospholipid Phytosome (APLC) for Improved Solubility, in Vivo Bioavailability, and Antioxidant Potential. Eur. J. Pharm. Sci. 2017, 108, 36–49.
- Chen, F.P.; Chien, M.H. Phytoestrogens Induce Differential Effects on Both Normal and Malignant Human Breast Cells in Vitro. Climacteric 2014, 17, 682–691.
- Abd El-Fattah, A.I.; Fathy, M.M.; Ali, Z.Y.; El-Garawany, A.E.R.A.; Mohamed, E.K. Enhanced Therapeutic Benefit of Quercetin-Loaded Phytosome Nanoparticles in Ovariectomized Rats. Chem. Biol. Interact 2017, 271, 30–38.
- Cury Rodrigues, M.F.; Stotzer, U.S.; Domingos, M.M.; Deminice, R.; Shiguemoto, G.E.; Tomaz, L.M.; de Sousa, N.M.F.; Ferreira, F.C.; Leite, R.D.; Selistre-de-Araújo, H.S.; et al. Effects of Ovariectomy and Resistance Training on Oxidative Stress Markers in the Rat Liver. Clinics 2013, 68, 1247–1254.
- Singh, R.P.; Gangadharappa, H.V.; Mruthunjaya, K. Phytosome Complexed with Chitosan for Gingerol Delivery in the Treatment of Respiratory Infection: In Vitro and in Vivo Evaluation. Eur. J. Pharm. Sci. 2018, 122, 214–229.
- Rahmani, A.H.; al Shabrmi, F.M.; Aly, S.M. Active Ingredients of Ginger as Potential Candidates in the Prevention and Treatment of Diseases via Modulation of Biological Activities. Int. J. Physiol. Pathophysiol. Pharmacol. 2014, 6, 125–136.
- Singh, R.P.; Gangadharappa, H.V.; Narke, R.M.; Jadhav, P.V. Stability-Indicating rp-hplc Method Development for the Estimation of Gingerol. Int. Res. J. Pharm. 2017, 8, 56–61.
- Kubra, I.R.; Jaganmohanrao, L. An Overview on Inventions Related to Ginger Processing and Products for Food and Pharmaceutical Applications. Recent Pat. Food Nutr. Agric. 2012, 4, 31–49.
- Rani, R.; Kumar, S.; Dilbaghi, N.; Kumar, R. Nanotechnology Enabled the Enhancement of Antitrypanosomal Activity of Piperine against Trypanosoma Evansi. Exp. Parasitol. 2020, 219, 108018.
- Ferreira, C.; Soares, D.C.; Barreto-Junior, C.B.; Nascimento, M.T.; Freire-De-Lima, L.; Delorenzi, J.C.; Lima, M.E.F.; Atella, G.C.; Folly, E.; Carvalho, T.M.U.; et al. Leishmanicidal Effects of Piperine, Its Derivatives, and Analogues on Leishmania Amazonensis. Phytochemistry 2011, 72, 2155–2164.
- Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Piperine—The Bioactive Compound of Black Pepper: From Isolation to Medicinal Formulations. Compr. Rev. Food Sci. Food Saf. 2017, 16, 124–140.
- Pachauri, M.; Gupta, E.D.; Ghosh, P.C. Piperine Loaded PEG-PLGA Nanoparticles: Preparation, Characterization and Targeted Delivery for Adjuvant Breast Cancer Chemotherapy. J. Drug Deliv. Sci. Technol. 2015, 29, 269–282.
- Zhu, J.Y.; Zhang, C.Y.; Dai, J.J.; Rahman, K.; Zhang, H. Diterpenoids with Thioredoxin Reductase Inhibitory Activities from Jatropha Multifida. Nat. Prod. Res. 2017, 31, 2753–2758.
- Teng, Z.; Yuan, C.; Zhang, F.; Huan, M.; Cao, W.; Li, K.; Yang, J.; Cao, D.; Zhou, S.; Mei, Q. Intestinal Absorption and First-Pass Metabolism of Polyphenol Compounds in Rat and Their Transport Dynamics in Caco-2 Cells. PLoS ONE 2012, 7, e29647.
- Karakaya, S. Bioavailability of Phenolic Compounds. Crit. Rev. Food Sci. Nutr. 2004, 44, 453–464.
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and Bioefficacy of Polyphenols in Humans. I. Review of 97 Bioavailability Studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S.
- Alqahtani, M.S.; Al-Yousef, H.M.; Alqahtani, A.S.; Tabish Rehman, M.; AlAjmi, M.F.; Almarfidi, O.; Amina, M.; Alshememry, A.; Syed, R. Preparation, Characterization, and in Vitro-in Silico Biological Activities of Jatropha Pelargoniifolia Extract Loaded Chitosan Nanoparticles. Int. J. Pharm. 2021, 606, 120867.