Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Steam explosion is a widely used hydrothermal pretreatment method, also known as autohydrolysis, which has become a popular pretreatment method due to its lower energy consumption and lower chemical usage. Steam explosion (SE) technology is a new type of physicochemical modification technology of food raw materials. SE is a method that presses high-pressure and high-temperature steam into cell walls and plant tissues, applying the thermochemical action of high-temperature cooking coupled with the physical tearing action of instantaneous blasting.
- steam explosion
- dietary fiber
- modification
- promote dissolution
- application
1. Introduction
With the development of living and economic standards, people have placed more importance on healthy diet requirements by eating more plant-based food like fruits and vegetables, which helps to maintain a healthy lifestyle. Cereals, fruits, and vegetables and other plant-derived foods are rich in functional compounds such as vitamins and dietary fiber. However, at present, more than one-third of the fruits and vegetables and their processing byproducts are not fully processed and utilized. These plant resources are usually treated by animal feed, landfill or incineration, and producers usually pay a certain price for it [1][2]. Thus, this inefficient processing of plant-derived food potentially has negative impacts on the environment.
Dietary fiber (DF) is a class of carbohydrate polymers that are neither digested nor absorbed in the small intestine, comprised of cellulose, hemicellulose, pectin, algae, and lignin. Dietary fiber mainly exists in the tough wall layer of plant cells. According to relative water solubility, dietary fiber can be divided into soluble and insoluble forms. These substances make up the hulls of cereal and wheat; and the roots, skin, stems and leaves of vegetables and fruits, which play an important beneficial physiological role. Over the years, research regarding the potential health benefits of dietary fiber in disease prevention has received considerable attention; obesity, type II diabetes, and cardiovascular diseases have been extensively studied [3]. Many previous studies found that there is an inverse relationship between dietary fiber intake and change in body weight. Koh-Banerjee et al. [4] supported this statement in a study that for every 40 g/d increase in whole grain intake, weight gain decreased by 1.1 lbs. A majority of studies show a positive correlation between high glycemic foods and type II diabetes. However, Meyer et al. [5] found that there was a strong inverse relationship between dietary fiber intake and diabetes. In the study, women who consumed an average of 26 g/d of dietary fiber had a 22% decreased risk of getting diabetes than women who consumed just 13 g/d. Recent studies suggest that increasing levels of dietary fiber may improve carbohydrate metabolism in a non–pharmacological way, resultingly having a positive effect on weight control and diabetes prevention. High quality DF should include more than 10% SDF and have favorable processing properties, as well as physiological activity and a healthcare function. Although the amount of total dietary fiber is fairly high in many plants, the content of SDF is only approximately 3–4% of the total dietary fiber. Insoluble dietary fiber may have some outstanding problems, such as rough taste, poor water-holding capacity, poor swelling power and weak functional activity; consequently, the utilization of this kind of raw material is not high [6]. It is urgent to carry out cost-effective industrial treatment of these resources and transform as much insoluble dietary fiber into soluble fiber as possible, which means modifying the dietary fiber raw materials in an efficient green way, in order to make better use of these resources.
As for the high-value utilization of raw materials rich in dietary fiber, pretreatment is needed to destroy their relatively dense physical structural barrier, so as to promote the efficient extraction, transformation, and utilization of DF [7]. At present, dietary fiber modification strategies may be classified into four types: physical, chemical, biological and combined method [7]. The glycosidic bond of DF is melted or broken using a physical method that makes use of high temperature, high pressure, immediate pressure decrease, explosion, high-speed impact, and shearing. This accomplishes the goal of modification, such as in steam explosion (SE) [8], ultrafine comminution (UC) [9], ultrasound [10], microwave [11], etc. The chemical method alters the structural and functional characteristics of DF by chemical reactions, such as alkaline hydrogen peroxide (AHP) treatment, alkali treatment, acid treatment, and Na2HPO4 treatment [12][13]. Compared with the physical method, the chemical method shows a short processing time and can react at room temperature. However, after the chemical method treatment, the modified DF exhibited low purity, and was prone to produce harmful components [14]. Utilizing certain enzymes or microorganisms to enzymatically hydrolyze or ferment raw materials with the goal of changing the content and bioactivity of DF is known as the biological method [15][16]. Biological methods are widely used in the modification of DF due to their high specificity, and benefits of milder processing conditions and environmental friendliness. The disadvantages of biological methods might be the high cost of enzyme purification and strain breeding [7]. Combining two or more ways to modify the DF is referred to as a combined modification method. Since the biological method requires a mild environment, the conditions of chemical methods are very harsh. Overall, the physical method is the main choice to combine with the other three methods [17][18]. Among these modification methods of DF, the physical methods are frequently used in the modification of dietary fiber because their advantages of low cost, short time consumption, simple operation, and lack of toxic waste generation [7].
Steam explosion (SE) technology, as a new type of physicochemical modification technology of food raw materials, has increasingly been studied by more and more researchers in recent years. SE is a method that presses high-pressure and high-temperature steam into cell walls and plant tissues, applying the thermochemical action of high-temperature cooking coupled with the physical tearing action of instantaneous blasting [8][19][20]. The physicochemical properties of the macromolecules of the fibrous raw materials are changed, thereby promoting the subsequent separation and conversion of solid-phase multi-component materials, which entails heating the biomass under pressurized steam to explode the cellulose fibrils and depolymerize lignin. At present, the steam explosion pretreatment has been used for ethanol production from straw materials, such as switchgrass and sugarcane bagasse [21], corn stover [22] and sunflower stalks [23]. In addition, the wall-breaking effect of SE on natural products has been widely studied. To sum up, this method has been gradually applied to the processing and modification of dietary fiber raw materials due to its low energy consumption and chemical usage [24][25].
2. Source and Classification of Dietary Fiber in Food
In the 1950s, Eben Hipsley [26] first proposed the definition of dietary fiber (DF), which described the food components from the cell walls of plants, and many scholars have since carried out research on DF. As suggested by Trowell et al. [27], DF was characterized as lignin-resistant and plant polysaccharides to hydrolyze human digestive enzymes. The American Society of Cereal Chemists includes oligosaccharides (DP 3–9) in the DF definition, as these substances have some physiological characteristics with the majority of DF. According to the AACC, DF is the edible section of plants, or carbohydrate-like substances, that are difficult for humans to digest and absorb in the small intestine; but are fully or partially fermented in the large intestine [28]. Polysaccharides, lignin, and associated plant matter are all included in DF. This was the first time that DF-related substances, such as phenolic compounds, were included in the definition of DF [28]. In 2009, the Codex Alimentarius Commission defined DF as 10 or more monomeric units of carbohydrate polymers (whether to include 3 or 9 monomer chain carbohydrates is determined by the national authority) that are not hydrolyzed by the endogenous enzymes in the human small intestine and fall into one of the following categories: (1) edible carbohydrate polymers naturally occurring in the food as consumed; (2) synthetic carbohydrate polymers or carbohydrate polymers that have been obtained from food raw material by physical, enzymatic, or chemical means and which have been shown to have a physiological effect or benefit to health as demonstrated by generally accepted scientific evidence to competent authorities [29]. With the development of nutrition and other disciplines, dietary fiber (DF) is known as the seventh most important nutrient by the nutrition community, and plays an important role in human health. DF exists in most natural plants, such as fruits (16.74–91.24%), vegetables (6.53–85.19%), grains (9.76–69.20%) and so on [30]. Indeed, dietary fiber is a heterogeneous complex of components with different physical, chemical, and physiological properties, which complicates direct analytical measurements [31]. DF is an important component of a healthy diet; the positive correlation with human health has been established by the scientific community [32]. More than 50% of functional foods in the market contain DF as the active ingredient [33]. Additionally, DF has many biologically active substances that are beneficial for our health, such as improving the intestinal flora, lowering blood glucose, decreasing the probability of obesity and cardiovascular disease, reducing the risk of some cancers, increasing fecal volume, promoting bowel movements, etc. [26][34][35][36][37].
The Chinese Nutrition Society defines DF as a carbohydrate polymer that is not easily digested by digestive enzymes, mainly from the plant cell wall, and comprised of cellulose, hemicelluloses, lignin, pectin and resin; more specifically defined as follows: (1) Cellulose is a long-chain polymer composed of glucose linked by β-1,4 glycosidic bonds; (2) Hemicellulose is a polymer composed of a mixture of monosaccharides such as arabinose, galactose, and xylose; (3) Lignin is not a polysaccharide, but a polymer composed of phenylpropane units; (4) Pectin is a polymer composed of uronic acid residues with rhamnose and contains neutral sugar branched chains; (5) Mucus and gums are mostly hemicelluloses. The existence of bioactive substances linked to the cell wall has a significant impact on the physicochemical characteristics of DF and also affects its physiological properties in humans. Additionally, according to the DF definition supplied by the AACC, phenolic compounds were included in the definition of DF. These components may play a significant role in DF properties, even though the properties are generally attributed to those of polysaccharides [33]. Goñi et al. [38] found that polyphenols appear in both fractions of fiber, but in a greater proportion in the insoluble portion. Consequently, polyphenols are an essential component of DF, and the definition of DF restricted to non-digestible polysaccharides and lignin could be extended to include polyphenols [39]. According to the solution properties of DF, they can be divided in two types: insoluble dietary fiber (IDF) and soluble dietary fiber (SDF) [40]. Additionally, the degree of fermentation in the colon can be divided into complete fermentation fibers (mostly SDF) and partial fermentation fibers (mostly IDF). SDF is mainly fermented by bacteria in the ileum and ascending colon, while IDF is primarily fermented in the distal colon. Many kinds of natural DF tend to have high IDF content and low SDF content, resulting in the rough taste of raw materials; poor functional properties such as water-holding capacity (WHC), oil-holding capacity (OHC), and swelling capacity (SC); and low physiological activity, which have seriously limited the development and utilization of dietary fiber resources [7]. Thus, finding the most appropriate modification method to enhance the yield and functional properties of DF is becoming the hot topic in the food processing research fields.
3. Effect of SE on the Soluble Modification of DF
In accordance with the principle of SE technology, this technology is very suitable for DF modification processing. SE technology is commonly used for the dietary fiber modification of agricultural products such as fruits and vegetables, grains, edible fungi, and aquatic products. Its typical characteristic is that the IDF in the materials will be moderately reduced, but the SDF content increased. With the further increase of steam explosion pressure and pressure holding time, the macromolecular polysaccharides will be degraded or depolymerized to different degrees [41]. Thus, the content of DF will increase first and then decrease. The modification conditions of SE technology in grains, fruits, and vegetables and yield changes in DF are shown in Table 1. When the SE strength was 1.5 MPa for 30 s, the SDF content from okara increased to 36.28%, 26 times higher compared with the control [41]. Additionally, with appropriate steam explosion conditions, the yield of SDF in raw materials, orange peel, soybean hulls, and sweet potato residue will significantly increase [42].
Steam explosion has the characteristics of both the mechanical modification and thermal modification of fibrous materials. The structure of high-fiber raw material was modified by the effects of acid-like hydrolysis, thermal degradation, mechanical rupture, hydrogen bond rupture, and structural rearrangement generated in the SE process. On the one hand, the interlaced lignin, cellulose, and hemicelluloses, staggered distributed in lignocellulosic biomass structures, were separated to a maximum extent by SE. Through SE technology, the physical structure was destroyed, loose microstructure and honeycomb-like porous structure obtained, and the specific surface area increased, thereby improving the extraction efficiency of SDF. On the other hand, due to the destruction of the lignin, cellulose and hemicelluloses structures, the cell wall structure was disintegrated, resulting in improving the yield of SDF through thermochemical degradation that converts IDF to SDF. This is mainly achieved because steam explosion can remove a large amount of hemicelluloses from fibrous raw materials [43]. In addition, the high-pressure thermoacidic environment during the steam explosion process led to massive hydrolysis of hemicelluloses on the cell wall, and the lignin wrapped around cellulose were hydrolyzed and softened. Additionally, SE was able to readily break the hydrogen bonds of the starch structure, leading the starch to partially degrade and gelatinize; the advanced spatial structure of the protein shifted to denaturation, all of which resulted in the conversion of IDF to SDF [44]. With the increase in steam explosion strength, the conversion trends become more obvious. The results of steam explosion of lignocellulosic biomass materials show that the removal rate of hemicelluloses can reach more than 85%. The principle is shown in Figure 1 [45].
Table 1. Effect of Steam explosion on the dietary fiber content of different food raw materials.
| Material Category | Steam Explosion Conditions | DF Content Changes | Reference Documentation |
|---|---|---|---|
| Wheat bran | 160 °C, 5 min | Water extractable arabinoxylan content was increased from 0.75% to 2.06%. | [46] |
| 0.8 MPa, 5 min | The SDF content increased to 9.62 g/100 g, which is 2.08-fold higher than that of the untreated. | [44] | |
| Okara | 1.5 MPa, 30 s | SDF content increased to 36.28%, higher by 26 times compared with the control okara. | [41] |
| Soybean hulls | 1.2 MPa, 180 s | The SDF yield rate was 11.12%. | [42] |
| Sweet potato residue | 0.35 MPa, 122 s | The SDF yield rate was increased from 3.81 ± 0.62% to 22.59 ± 0.35%. | [47] |
| Rosa roxburghii pomace | 0.87 MPa, 97 s | The SDF content was increased from 9.31 ± 0.07% to 15.82 ± 0.31%. | [48] |
| Grapefruit peel | 0.8 MPa, 90 s | The pectin yield rate reached 17.5%. | [49] |
| Orange peel | 0.8 MPa, 7 min, combined with 0.8% sulfuric-acid soaking | SDF was increased from 8.04% to 33.74% in comparison with the control. | [17] |
| Apple pomace | 0.51 MPa, 168 s, sieving mesh size, 60. | The SDF yield from apple pomace after SE was 29.85%, which is 4.76 times the yield of SDF (6.27%) in untreated apple pomace. | [50] |
| Okra seed | 1.5 MPa, 5 min | The SDF content reached 6.5%. | [51] |
| Ampelopsis grossedentata | 0.4 MPa, 4 min | The yield of crude polysaccharides reached 5.35 ± 0.12%, which is an increase of 2.2 times over that of the materials without SE pretreatment. | [52] |
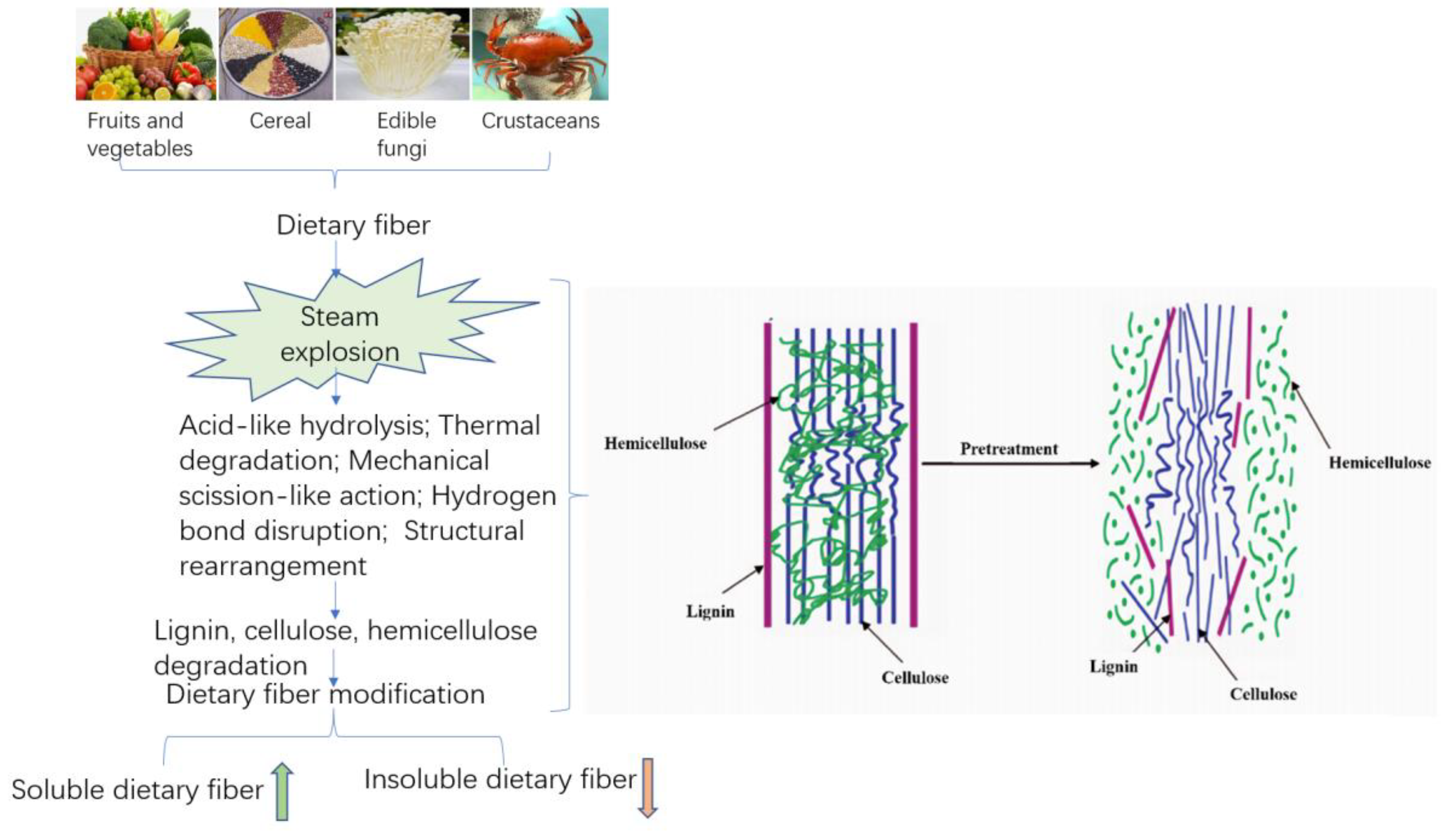
Figure 1. Schematic diagram of the modification mechanism of dietary fiber in Steam explosion.
This entry is adapted from the peer-reviewed paper 10.3390/foods11213370
References
- Angulo, J.; Mahecha, L.; Yepes, S.A.; Yepes, A.M.; Bustamante, G.; Jaramillo, H.; Valencia, E.; Villamil, T.; Gallo, J. Nutritional evaluation of fruit and vegetable waste as feedstuff for diets of lactating Holstein cows. J. Environ. Manag. 2012, 95, S210–S214.
- Leroy, B.; Bommele, L.; Reheul, D.; Moens, M.; Neve, S.D. The application of vegetable, fruit and garden waste (VFG) compost in addition to cattle slurry in a silage maize monoculture: Effects on soil fauna and yield. Eur. J. Soil Biol. 2007, 43, 91–100.
- Telrandhe, U.B.; Kurmi, R.; Uplanchiwar, V.; Mansoori, M.H.; Jain, S.K. Nutraceuticals—A Phenomenal Resource in Modern Medicine. Int. J. Univers. Pharm. Life Sci. 2012, 2, 179–195.
- Koh-Banerjee, P.; Franz, M.; Sampson, L.; Liu, S.; Jacobs, D.R.; Spiegelman, D.; Willett, W.; Rimm, E. Changes in whole-grain, bran, and cereal fiber consumption in relation to 8-y weight gain among men. Am. J. Clin. Nutr. 2004, 80, 1237–1245.
- Meyer, K.A.; Kushi, L.H.; Jacobs, D.R.; Slavin, J.; Sellers, T.A.; Folsom, A.R. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am. J. Clin. Nutr. 2000, 71, 921–930.
- Liu, M.; Jiang, Y.; Zhang, L.; Wang, X.; Lin, L. Effect of Steam Explosion Modification and in Vitro Simulated Digestion on Antioxidant Capacity of Dietary Fiber of Pineapple Peel, IOP conference series. Earth Environ. Sci. 2019, 330, 42055.
- Gan, J.; Xie, L.; Peng, G.; Xie, J.; Chen, Y.; Yu, Q. Systematic review on modification methods of dietary fiber. Food Hydrocoll. 2021, 119, 106872.
- Chandra, R.P.; Bura, R.; Mabee, W.E.; Berlin, A.; Saddler, J.N. Substrate Pretreatment: The Key to Effective Enzymatic Hydrolysis of Lignocellulosics. Adv. Biochem. Eng. Biotechnol. 2007, 108, 67–93.
- Guoyong, Y.; Jia, B.; Jing, Z.; Quanhong, L.; Chen, C. Modification of carrot (Daucus carota Linn. var. Sativa Hoffm.) pomace insoluble dietary fiber with complex enzyme method, ultrafine comminution, and high hydrostatic pressure. Food Chem. 2018, 257, 333–340.
- Liurong, H.; Xiaona, D.; Yunshu, Z.; Yuxiang, L.; Haile, M. Modification of insoluble dietary fiber from garlic straw with ultrasonic treatment. J. Food Process Pres. 2018, 42, e13399.
- Enwei, W.; Rui, Y.; Hepeng, Z.; Penghui, W.; Suqing, Z.; Wanchen, Z.; Yang, Z.; Hongli, Z. Microwave-assisted extraction releases the antioxidant polysaccharides from seabuckthorn (Hippophae rhamnoides L.) berries. Int. J. Biol. Macromol. 2019, 123, 280–290.
- Yuge, N.; Na, L.; Qi, X.; Yuwei, H.; Guannan, X. Comparisons of three modifications on structural, rheological and functional properties of soluble dietary fibers from tomato peels. LWT 2018, 88, 56–63.
- Kunli, W.; Mo, L.; Yuxiao, W.; Zihao, L.; Yuanying, N. Effects of extraction methods on the structural characteristics and functional properties of dietary fiber extracted from kiwifruit (Actinidia deliciosa). Food Hydrocoll. 2021, 110, 106162.
- Suya, H.; Yawen, H.; Yanping, Z.; Zhuang, L. Modification of insoluble dietary fibres in soya bean okara and their physicochemical properties. Int. J. Food Sci. Technol. 2015, 50, 2606–2613.
- Chaofan, W.; Rongzhen, S.; Siqing, W.; Wenliang, W.; Feng, L.; Xiaozhen, T.; Ningyang, L. Modification of insoluble dietary fiber from ginger residue through enzymatic treatments to improve its bioactive properties. LWT 2020, 125, 109220.
- Mengyun, J.; Jiajun, C.; Xiaozhen, L.; Mingyong, X.; Shaoping, N.; Yi, C.; Jianhua, X.; Qiang, Y. Structural characteristics and functional properties of soluble dietary fiber from defatted rice bran obtained through Trichoderma viride fermentation. Food Hydrocoll. 2019, 94, 468–471.
- Lei, W.; Honggao, X.; Fang, Y.; Rui, F.; Yanxiang, G. Preparation and physicochemical properties of soluble dietary fiber from orange peel assisted by steam explosion and dilute acid soaking. Food Chem. 2015, 185, 90–98.
- Jiapan, G.; Ziyan, H.; Qiang, Y.; Guanyi, P.; Yi, C.; Jianhua, X.; Shaoping, N.; Mingyong, X. Microwave assisted extraction with three modifications on structural and functional properties of soluble dietary fibers from grapefruit peel. Food Hydrocoll. 2020, 101, 105549.
- Auxenfans, T.; Cr Nier, D.; Chabbert, B.; Pa, S.G. Understanding the structural and chemical changes of plant biomass following steam explosion pretreatment. Biotechnol. Biofuels 2017, 10, 36.
- Sarker, T.R.; Pattnaik, F.; Nanda, S.; Dalai, A.K.; Meda, V.; Naik, S. Hydrothermal pretreatment technologies for lignocellulosic biomass: A review of steam explosion and subcritical water hydrolysis. Chemosphere 2021, 284, 131372.
- Shannon, E.; Renata, B. The effect of biomass moisture content on bioethanol yields from steam pretreated switchgrass and sugarcane bagasse. Bioresour. Technol. 2011, 102, 2651–2658.
- Yanling, Y.; Yujie, F.; Chen, X.; Jia, L.; Dongmei, L. Onsite bio-detoxification of steam-exploded corn stover for cellulosic ethanol production. Bioresour. Technol. 2011, 102, 5123–5128.
- Vaithanomsat, P.; Chuichulcherm, S.; Apiwatanapiwat, W. Bioethanol production from enzymatically saccharified sunflower stalks using steam explosion as pretreatment. World Acad. Sci. Eng. Technol. 2009, 49, 140–143.
- Balat, M. Production of bioethanol from lignocellulosic materials via the biochemical pathway: A review. Energ. Convers. Manag. 2011, 52, 858–875.
- Maria, C.D.A.W.; Carlos, M.; George, J.D.M.R.; Ester, R.G. Increase in ethanol production from sugarcane bagasse based on combined pretreatments and fed-batch enzymatic hydrolysis. Bioresour. Technol. 2013, 128, 448–453.
- Brownlee, I.A. The physiological roles of dietary fibre. Food Hydrocoll. 2011, 25, 238–250.
- Trowell, H.; Southgate, D.A.; Wolever, T.M.; Leeds, A.R.; Jenkins, D.J. Letter: Dietary fibre redefined. Lancet 1976, 307, 967.
- Paola, V.; Aurora, N.; Vincenzo, F. Cereal dietary fibre: A natural functional ingredient to deliver phenolic compounds into the gut. Trends Food Sci. Tech. 2008, 19, 451–463.
- Lupton, J.R.; Betteridge, V.A.; Pijls, L.J. Codex final definition of dietary fibre: Issues of implementation. Qual. Assur. Saf. Crops Foods 2009, 1, 206–212.
- Yang, H.; Bixiang, W.; Liankui, W.; Fengzhong, W.; Hansong, Y.; Dongxia, C.; Xin, S.; Chi, Z. Effects of dietary fiber on human health. Food Sci. Hum. Wellness 2022, 11, 1–10.
- Susanne, W.; Kommer, B.; Jan-Willem, V.D.K. Dietary fibre: Challenges in production and use of food composition data. Food Chem. 2013, 140, 562–567.
- Melissa, M.K.; Michael, J.M.; Gregory, G.F. The health benefits of dietary fiber: Beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metabolism 2012, 61, 1058–1066.
- Macagnan, F.T.; Da Silva, L.P.; Hecktheuer, L.H. Dietary fibre: The scientific search for an ideal definition and methodology of analysis, and its physiological importance as a carrier of bioactive compounds. Food Res. Int. 2016, 85, 144–154.
- Jinjin, C.; Qingsheng, Z.; Liwei, W.; Shenghua, Z.; Lijun, Z.; Bing, Z. Physicochemical and functional properties of dietary fiber from maca (Lepidium meyenii Walp.) liquor residue. Carbohyd. Polym. 2015, 132, 509–512.
- Bader, U.A.H.; Saeed, F.; Khan, M.A.; Niaz, B.; Rohi, M.; Nasir, M.A.; Tufail, T.; Anbreen, F.; Anjum, F.M. Modification of barley dietary fiber through thermal treatments. Food Sci. Nutr. 2019, 7, 1816–1820.
- Shuai, L.; Mengyun, J.; Jiajun, C.; Haisheng, W.; Ruihong, D.; Shaoping, N.; Mingyong, X.; Qiang, Y. Removal of bound polyphenols and its effect on antioxidant and prebiotics properties of carrot dietary fiber. Food Hydrocoll. 2019, 93, 284–292.
- Ji, Y.S.; Young-Min, K.; Byung-Hoo, L.; Sang-Ho, Y. Increasing the dietary fiber contents in isomaltooligosaccharides by dextransucrase reaction with sucrose as a glucosyl donor. Carbohyd. Polym. 2020, 230, 115607.
- Isabel, G.; Díaz-Rubio, M.E.; Jara, P.; Fulgencio, S. Towards an updated methodology for measurement of dietary fiber, including associated polyphenols, in food and beverages. Food Res. Int. 2009, 42, 840–846.
- Saura-Calixto, F. Concept and health-related properties of nonextractable polyphenols: The missing dietary polyphenols. J. Agr. Food Chem. 2012, 60, 11195–11200.
- Xiaoran, D.; Suyan, G.; Shoumin, J.; Litian, Z.; Ruihuan, D.U.; Aili, X.; Biao, Q.I.; Lei, W. Research Status and Progress on Modification of Dietary Fiber at Home and Abroad. Agric. Biotechnol. 2019, 8, 131–135.
- Li, B.; Yang, W.; Nie, Y.; Kang, F.; Goff, H.D.; Cui, S.W. Effect of steam explosion on dietary fiber, polysaccharide, protein and physicochemical properties of okara. Food Hydrocoll. 2019, 94, 48–56.
- Zhu, L.; Yu, B.; Chen, H.; Yu, J.; Yan, H.; Luo, Y.; He, J.; Huang, Z.; Zheng, P.; Mao, X.; et al. Comparisons of the micronization, steam explosion, and gamma irradiation treatment on chemical composition, structure, physicochemical properties, and in vitro digestibility of dietary fiber from soybean hulls. Food Chem. 2021, 366, 130618.
- Glasser, W.G.; Wright, R.S. Steam-assisted biomass fractionation. II. fractionation behavior of various biomass resources. Biomass Bioenerg. 1998, 14, 219–235.
- Sui, W.; Xie, X.; Liu, R.; Wu, T.; Zhang, M. Effect of wheat bran modification by steam explosion on structural characteristics and rheological properties of wheat flour dough. Food Hydrocoll. 2018, 84, 571–580.
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729.
- Aktas-Akyildiz, E.; Mattila, O.; Sozer, N.; Poutanen, K.; Koksel, H.; Nordlund, E. Effect of steam explosion on enzymatic hydrolysis and baking quality of wheat bran. J. Cereal. Sci. 2017, 1, 25–32.
- Wang, T.; Liang, X.; Ran, J.; Sun, J.; Jiao, Z.; Mo, H. Response surface methodology for optimisation of soluble dietary fibre extraction from sweet potato residue modified by steam explosion. Int. J. Food Sci. Technol. 2017, 52, 741–747.
- Zhai, X.; Ao, H.; Liu, W.; Zheng, J.; Li, X.; Ren, D. Physicochemical and structural properties of dietary fiber from Rosa roxburghii pomace by steam explosion. J. Food Sci. Technol. 2022, 59, 2381–2391.
- Zhao, B.; Wang, C.; Zeng, Z.; Xu, Y. Study on the extraction process of pectin from grapefruit peel based on steam explosion technology. IOP Conf. Ser. Earth Environ. Sci. 2021, 792, 12008.
- Liang, X.; Ran, J.; Sun, J.; Wang, T.; Jiao, Z.; He, H.; Zhu, M. Steam-explosion-modified optimization of soluble dietary fiber extraction from apple pomace using response surface methodology. CyTA J. Food 2018, 16, 20–26.
- Hu, L.; Guo, J.; Zhu, X.; Liu, R.; Wu, T.; Sui, W.; Zhang, M. Effect of steam explosion on nutritional composition and antioxidative activities of okra seed and its application in gluten-free cookies. Food Sci. Nutr. 2020, 8, 4409–4421.
- Liu, C.; Sun, Y.; Jia, Y.; Geng, X.; Pan, L.; Jiang, W.; Xie, B.; Zhu, Z. Effect of steam explosion pretreatment on the structure and bioactivity of Ampelopsis grossedentata polysaccharides. Int. J. Biol. Macromol. 2021, 185, 194–205.
This entry is offline, you can click here to edit this entry!
