Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell Biology
Phase separation is a process by which a well-mixed solution of macromolecules such as proteins or nucleic acids spontaneously separates into two phases: a dense phase and a dilute phase.
- phase separation
- membraneless organelle
- biomolecular condensates
1. Introduction
Biological evolution is the evolutionary process of the development of all life forms, and an important characteristic is the progressive complexity and refinement of the different levels of morphological structures. Typically, different biological reactions take place in different organelles in an orderly manner; for example, transcription occurs primarily in the nucleus, protein modification occurs in the endoplasmic reticulum and Golgi, and molecular degradation occurs in lysosomes. These organelles are surrounded by a single or double layer of molecular membranes and are isolated from the surrounding environment. To ensure that various cellular components can aggregate at the correct time and in the proper space to perform their corresponding functions, cellular molecules are isolated in different cellular compartments as needed. In addition to classic membrane-bound organelles, much evidence further suggests that cells possess various membraneless compartments, including the nucleolus, Cajal body, and PcG body in the cellular nucleus [1], along with stress granules (SGs) and P-bodies in the cytoplasm [2,3,4,5]. Proteins, RNA, and other molecular components constitute these membraneless organelles, and phase separation drives the processes involved [2,6]. Although not covered by cell membranes, membraneless organelles are still capable of frequent molecular exchange with the environment. With ongoing scientific inquiry, many insights have been gained into the structure of these organelles. However, questions about why these organelles form, the mechanisms involved, and how their biological properties affect their function have not been answered. As science has advanced, these questions have begun to be resolved, and a deeper understanding of the organization, molecular properties, and regulation of membraneless organelles has emerged [7,8]. In recent years, there has been growing evidence that membraneless organelles are involved in the development of multiple cancers [9,10]. These findings have given rise to a new domain of cellular biology. The emphasis is on learning how cell substances are organized into membraneless organelles, how to promote their activity, and how disorders in these organelles frequently lead to diseases, encouraging researchers to consider the biological processes involved from the viewpoint of phase separation.
Cancer is a disease that seriously threatens human health. Both in terms of morbidity and mortality, cancer is a major global public health problem and second only to cardiovascular disease in terms of mortality [11]. Although research on the occurrence and development of cancer has a history spanning many years, its treatment is still a great problem facing the world. To overcome this challenge, new concepts are urgently needed to characterize and explain the complicated mechanisms of human cancer. An increasing body of evidence suggests that there is a close link between abnormal phase separation condensation assembly and aberrant oncogenic procedures. The advent of protein phase separation offers a novel possibility for targeting refractory cancer.
2. Overview of Phase Separation
Phase separation is a process by which a well-mixed solution of macromolecules such as proteins or nucleic acids spontaneously separates into two phases: a dense phase and a dilute phase [12]. Whether phase separation occurs in a solution depends greatly on the concentration and properties of the macromolecules and the solution, as well as on the environmental conditions [13]. With a growing understanding of the basic molecular principles of phase separation, there is an awareness of the different functions of phase separation in various cellular processes. In general, the main ones include stress response, regulation of gene expression and control of signal transduction [6,14], protein degradation [15], cytoskeleton assembly [16], and gene activation [17] or repression [18], including epigenetics, transcription, and translation. Furthermore, it has been demonstrated that many fundamental biological processes are inseparable from phase separation, including heterochromatin formation [18,19], nucleocytoplasmic transport [20], supramolecular assemblies [21], and assembly of membraneless compartments, such as SGs [22]. Cell architectures formed by phase separation are named biomolecular condensates to mirror their provenance through condensation reactions [2,23]. Contrary to other types of components, they can enrich molecules, and rapid exchange of components and agglomeration of droplets can form specific cellular structures called membraneless organelles by phase separation. In different physiopathological situations, biomolecular condensates can be converted into different states of matter. Similarly, condensates play an essential role in the life activities of various organisms, including advanced structures, gene expression regulation [24], autophagic degradation of incorrectly folded or unneeded proteins [25], signal cluster assembly, and synaptic plasticity regulation of the formation of signaling molecules [10].
2.1. Phase Separation to Form Membraneless Organelles
In 2009, Hyman and Brangwynne found that some properties of P granules resemble liquids and that regulated dissolution/condensation drives their localization, and the researchers first realized that membraneless organelles may be driven by phase separation [26]. Membraneless organelles are dynamic structures with liquid-like physical properties [26]. Due to a lack of lipid-rich membranes, changes in the surrounding environment can easily affect their internal homeostasis, so the protein composition and morphology of membraneless organelles respond accordingly to changes in the cellular environment, and this ability may represent the mechanism underlying the stress response sensed by membraneless organelles [27]. For example, oncogenic ARF protein is localized in the nucleolus and released into the nucleoplasm through changes in phase separation in response to environmental stresses of DNA damage and oncogene activation, hence activating the p53 oncogenic pathway [28].
What is the unique role of membraneless organelles driven by phase separation? The nucleolus is the largest and most intensively studied membraneless organelle, serving as the center of ribosomal RNA synthesis and nascent ribosome assembly in eukaryotic cells. Ribosome biogenesis is vectorial, starting from fibrillar centers (FCs), where rDNA is transcribed into rRNA [29]. Paraspeckles are located in chromatin gaps and are subnuclear bodies built on NEAT1 (a long noncoding RNA). Paraspeckles are involved in many physiological processes, including the cellular stress response, cell differentiation, corpus luteum formation, and cancer development [30]. The proteins that comprise paraspeckles are related to RNA polymerase II transcription and RNA processing. Cajal bodies (CBs) and histone locus bodies (HLBs) could be responsive to stress conditions, and they are nuclear bodies (NBs) involved in the transcriptional and posttranscriptional regulation of small nuclear RNAs and histone genes [31]. With the deepening of research, an increasing number of membraneless organelles and their functions are being discovered (Figure 1).
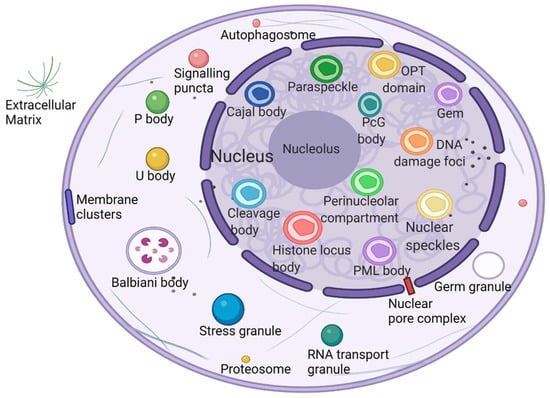
Figure 1. Biomolecular condensates located throughout the nucleus and cytoplasm. Created with BioRender.com.
2.2. Multivalent Interactions Promote the Formation of a Phase Separation Network
Recent studies have shown that biomolecular phase separation occurs through multivalency or the capacity to participate in weak multivalent effects that rapidly assemble, disconnect, and recombine. These multiple interactions are facilitated by proteins that embrace multiple folding module domains or intrinsically disordered regions (IDRs) [32] or oligomerization domains [33]. Another type of phase separation protein contains polymeric structural fields, such as DIX domains, which can cross-link to form three-dimensional condensates [34]. These multivalent interactions mainly include two types: one class of intracellular interactions, such as protein–protein, protein–RNA, and RNA–RNA interactions; and another class of weak, instant, multivalent interplay between IDRs, consisting of π–π interactions, cation–anion interactions, dipole–dipole interactions, and π–cation interactions [10,35,36].
In addition, another mechanism, called bridging-induced phase separation (BIPS), has been demonstrated in a number of chromatin-related phase separation phenomena. It has been confirmed that BIPS is the basis for DNA-mediated clustering cohesion [37]. Local bridging along distal segments of DNA molecules is an essential element of BIPS and a characteristic of BIPS that does not exist in other forms of phase separation [38]. In this way, phase separation is induced between chromatin regions (stable or transient) that interact with different types of bridging factors [38].
How do cells use phase separation to respond to changes in cell surroundings? Cells construct and regulate dynamic membraneless organelles through characteristics encoded in intrinsically disordered proteins (IDPs) of related proteins, many of which play central functions in a variety of cell features. IDPs are conformationally flexible, often interacting with their bonding companions via short sequence motifs that reappear within disordered areas, and this multivalent interplay is common in macromolecular complexes [39]. As T-cell receptor components form a cluster of membrane-associated phase separation signals, during T-cell activation, phase separation is driven by the multivalence of LAT, GRB2, SLP-76, Nck, and WASP [40].
3. Mechanisms of Phase Separation in Tumorigenesis
Incorrect or abnormal phase separation of biological macromolecules is closely related to the occurrence of many types of diseases, such as cancer, neurodegenerative diseases, and infectious diseases [41,42,43]. The formation and regulation of aberrant biomolecular condensates is changing the way people think about coping with many diseases, including tumor genesis, diagnosis, and treatment. Researchers are no longer considering highly recurrent point mutations in tumors merely based on the structural visual field but are also considering the condensates involved in these mutations. Generally, combined with current existing research, there are three mechanisms of abnormal biological phase separation leading to disease: (a) The first mechanism is biomacromolecule condensate dysregulation. In cancer, IDR-related signal receptor mutations or chromosome translocations can promote the shape of signal clusters or condensates at transcription or DNA damage repair sites and then change the cell signal cascade, drive abnormal transcription programs or DNA damage repair, and promote cancer cell proliferation [44]. (b) The second mechanism is the changing of the critical catalysts required for phase separation. There is evidence that enzymes can regulate the assembly of biomolecular condensates through posttranslational modification (PTM). For example, DYRK3 is located in condensate and phosphorylates several serine and threonine residue groups in IDRs [45]. During stress recovery, inactivation and activation of DYRK3 are crucial to the formation and dissolution of SG [46]. (c) The third mechanism is the altering of general physicochemical conditions in cells. Cells exposed to stress undergo extreme fluctuations in the levels of ion concentration, osmotic conditions, and pH values, which can change the solubility and interactions of biological macromolecules, resulting in abnormal phase separation [47] (Figure 2).
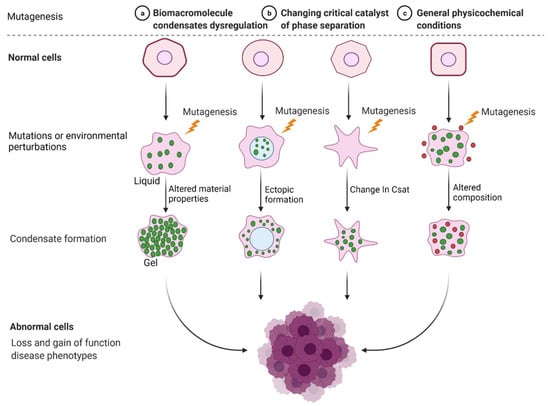
Figure 2. Mechanisms of abnormal phase separation in disease. Theoretical possibilities of how disease phenotypes arise from abnormal phase separation and condensate formation. Created with BioRender.com.
Cancer is the abnormal proliferation of cells in local tissues under the action of various tumorigenic factors in the body. In addition to having unlimited proliferation and multidirectional differentiation potential, many cancer cells have the ability to evade growth repression, engage in replicative immortality, avoid immune destruction, and cause instability of the genome [48], as well as newly discovered tumor features, such as unlocking phenotypic plasticity and reprogramming nonmutational epigenetics [49]. It is worth noting that gene mutations, tumor-promoting inflammation, unlocking phenotypic plasticity, and polymorphic microbiomes increase the possibility of tumors. Gene mutations in cancer often lead to oncogene activity imbalance or inactivation of tumor suppressor genes, thus promoting the carcinogenic process. Despite considerable advances in our understanding of how mutations promote the carcinogenic process, the exact pathogenesis of tumorigenesis remains unclear, as does the mechanism by which tumor cells acquire these features. Phase separation offers a new direction for understanding cancer phenotypes (Figure 3). Generally speaking, it can be divided into two aspects. Dysregulation is driven by phase separation itself. For example, interferon-γ improves tumor sensitivity to immunotherapy by inhibiting YAP phase separation [50]; phase separation of YAP and TAZ participates in activating EMT [51]; and increasing the formation of SGs overcomes stress-induced cancer cell death [52]. In addition to this, carcinogenicity can also be affected by the dysregulation of signaling proteins involved in phase separation. For instance, MYC forms transcription condensates by binding to superenhancers, which lead to VEGF expression and the promotion of angiogenesis [17]. In addition, aberrant phase segregation of the ENL protein can recruit a large number of associated transcription complexes, which lead to genomic rearrangements in cancer [53]. Fusion between promyelocytic leukemia protein (PML) bodies permit telomere lengthening and enable replicative immortality [54], and SPOP mutation inhibits the catabolism of prooncogenic substrates, thus escaping growth inhibition [55]. An increasing number of studies has shown that the process of phase separation cannot be ignored for the progression and treatment of human diseases.
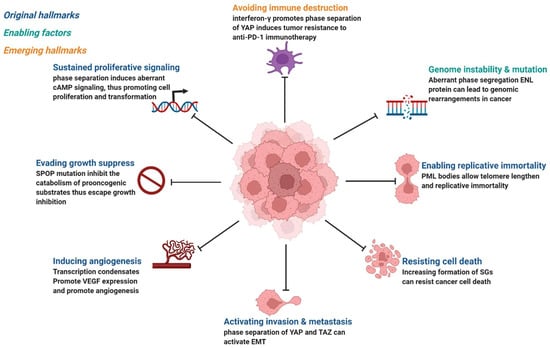
Figure 3. Phase separation abnormalities are involved in most of the processes known as cancer hallmarks. Created with BioRender.com.
Abnormality of phase separation may promote the occurrence of some cancers. For example, one study has directly linked protein phase separation to cancer. In vitro, substrates can trigger phase separation of speckle-type BTB/POZ protein (SPOP) and colocalization in membraneless organelles in cells, and carcinogenic mutations in cancer suppressor SPOP result from interference with phase separation and colocalization in membraneless organelles linked to specific phase separation defects [55]. Cullin3-ring ubiquitin ligase is associated with a variety of solid tumors, and SPOP as its substrate adapter is one of the first cancer-specific related proteins to undergo phase isolation [55,56]. Molecular pathologist Miguel Rivera found a protein associated with Ewing sarcoma. This protein can activate oncogene expression when it accumulates near the genome related to tumorigenesis, and abnormal “phase separation” may promote the aggregation of this protein near these regions, leading to the occurrence of Ewing sarcoma [57]. Moreover, the FUS/EWS/TAF15 (FET) fusion oncoprotein enhances abnormal gene transcription by site-specific phase separation and is an indispensable carcinogenic driver in various human cancers [58,59]. This study reveals that phosphatase protein can undergo phase separation, suggesting that phase separation is a notable means for cells to regulate phosphatase activity. Gene mutation can change the phase separation ability of protein and then change the protein function, leading to the occurrence of human diseases, highlighting the importance of phase separation in human disease occurrence and development [59]. The following is a summary of the various types of cancer condensate formation and the regulation of cancer-associated proteins (Table 1 and Figure 4).
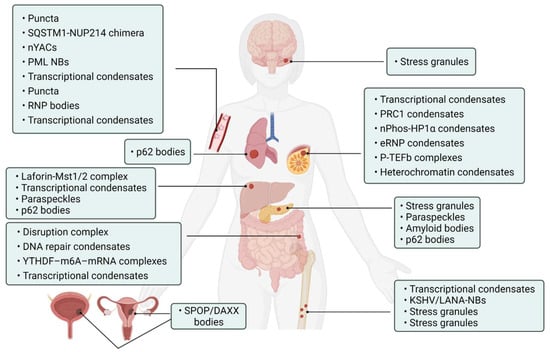
Figure 4. Cancer-related condensates in human cancers. Distribution of various types of cancer and related condensates in the human body. Created with BioRender.com.
Table 1. Condensates in cancer. Abnormal protein phase separations are involved in the progression of various cancers.
| Tumor Types | Proteins | Biomolecular Condensates | Biological Roles | References |
|---|---|---|---|---|
| Hepatocellular Carcinoma | YAP | Laforin-Mst1/2 complex | Block Hippo kinase and accelerate tumorigenesis | [60] |
| YAP, TAZ | Transcriptional condensates | Activate prevalently in cancer | [61,62] | |
| NEAT1_2 | Paraspeckles | Induce transcription of various gene sustained by cancer cells | [63] | |
| p62 | p62 bodies | Induce carcinogenesis | [64] | |
| Lung Cancer | KEAP1/NRF2/p62 | p62 bodies | Increase the risk of tumor genesis | [65] |
| Pancreatic Cancer | KRAS | Stress granules | Improve cancer cell suitability | [52,66] |
| p53 | Paraspeckles | Promote the expression of tumor suppressors | [67] | |
| ACM | Amyloid bodies | Promote tumor tissue growth | [68] | |
| p62 | p62 bodies | Critical in regulating tumorigenesis through autophagy | [69,70,71] | |
| Colorectal Cancer | APC | Disruption complex | Effective β-catenin degradation | [72,73] |
| 53BP1 | DNA repair condensates | Respond to DNA damage | [74] | |
| YTHDF1/2/3 | YTHDF–m6A–mRNA complexes | Weaken mRNA translation | [75,76] | |
| β-catenin | Transcriptional condensates | Wnt factor driving cancer | [77,78] | |
| Leukemia | NUP98 FOs | Puncta | Associated with malignant transformation of hematopoietic cells | [79] |
| NUP214 | SQSTM1-NUP214 chimera | Associated with malignant transformation of hematopoietic cells | [80] | |
| YTHDC1 | nYACs | Maintains mRNA stability and controls cancer cell survival and differentiation | [81] | |
| PML/RARA | PML NBs | Involved in oncogenic signaling | [82,83] | |
| MYB | Transcriptional condensates | Drive oncogenic TAL1 expression | [84] | |
| ENL | Puncta | Regulates oncogenic transcriptional program | [53,85] | |
| NPM1 | RNP bodies | Ribosome biosynthesis | [86,87] | |
| Leukemia/Sarcoma | FUS/TAF15 PLD | Transcriptional condensates | Drive aberrant tumorigenic transcriptional program | [88] |
| Sarcoma | KSHV/LANA | KSHV/LANA-NBs | Cause alterations in gene expression | [89,90] |
| FUS/CHOP | Stress granules | Carcinogenic transformation | [91] | |
| EWS/FLI1 | Transcriptional condensates | Promote gene transcription associated with Ewing’s sarcoma | [92] | |
| YB-1 | Stress granules | Cancer metastatic marker | [93] | |
| Medulloblastoma | DDX3X | Stress granules | Impair global translation | [94,95] |
| Breast Cancer | YAP/TAZ | Transcriptional condensates | Promote expression of target gene | [51,96] |
| CBX2 | PRC1 condensates | Gene suppression | [97,98,99] | |
| HP1α | nPhos-HP1α condensates | Epigenetic regulation | [18,19,100] | |
| ER | eRNP condensates | Synergistic assembly of activated chromosome enhancers | [101] | |
| P-TEFb | P-TEFb complexes | Activate and increase transcription of EMT transcription factors | [102,103] | |
| MeCP2 | Heterochromatin condensates | Chromosome maintenance and transcriptional silence | [104,105] | |
| Prostate/Endometrial cancer | SPOP | SPOP/DAXX bodies | Promote tumor development | [55,56] |
| Other cancers | PARP-1 | DNA damage condensates | Promote DNA damage | [106,107] |
| OCT4 | OCT4-MED1-IDR complex | Control gene transcription | [17,108] | |
| MED1, BRD4 | Transcriptional condensates | Activate gene transcription | [109,110,111] | |
| CDK7 | Transcriptional condensates | Kinase overexpression and targeting in cancer | [112,113] | |
| HSF1 | Transcriptional condensates | Act as “sensors” regulating cell fate | [114,115] | |
| Rad52 | Repair center condensates | DNA repair | [116] | |
| hnRPNA1, FUS, G3NP1/2 | Stress granules | Modulate the stress response | [117,118] | |
| FMRP/CAPRIN1 | FMRP-CAPRIN1 condensates | Control RNA process and translation | [119] |
This entry is adapted from the peer-reviewed paper 10.3390/cancers14235971
This entry is offline, you can click here to edit this entry!
