Vaccination has been identified as a critical method of disease control in the context of the current COVID-19 pandemic. This research updated information on vaccine development and to identify areas of concern that require further research. The researchers reviewed the literature on the development of COVID-19 vaccines, their efficacy, and use in special populations, as well as current vaccination strategies. To date, 170 vaccines are in clinical development, with 41 being already approved for use in various countries. The majority of vaccines approved for human use are vector-, subunit-, DNA-, or mRNA-based vaccines, or inactivated viruses. Because of the ongoing mutation of the SARS-CoV-2 virus, well-studied vector vaccines are losing relevance due to the ability of new virus strains to bypass neutralizing antibodies. Simultaneously, PS-based vaccines are becoming more popular. There is mounting evidence that the immunogenicity of COVID-19 vaccines is linked to their clinical efficacy. This has resulted in a shift in vaccination strategies, as well as the use of booster doses and revaccination. Furthermore, vaccination restrictions for children, pregnant women, the elderly, and people with chronic immunosuppressive diseases have been lifted, allowing more people to be vaccinated. New data on vaccine safety, including the incidence of serious adverse events, have been collected. Despite significant advances in the development of and research on COVID-19 vaccines, many questions remain that require further investigation.
- immunization
- prevention
- vector
- mRNA
- protein subunits
- COVID-19
1. Introduction
2. COVID-19 Vaccine Platforms and Their Characteristics
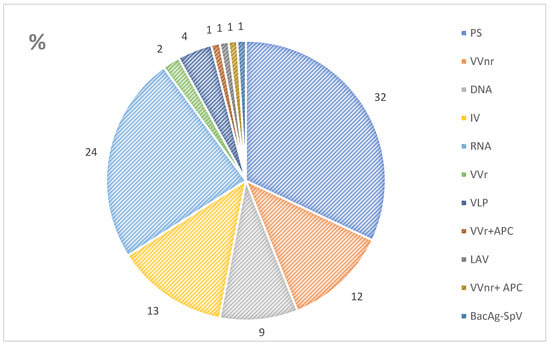
3. Immunogenicity and Safety of Vaccines
4. Vaccinations of Various Populations
4.1. General Population
COVID-19 mRNA vaccines (BNT162b2 and mRNA-1273) are recommended by the US Centers for Disease Control and Prevention (CDC) for primary and booster vaccinations in all populations. Currently, primary vaccination with one type of vaccine is recommended [19]. The WHO considers the following vaccines to be safe and effective: AZD1222; Ad26.COV2.S; mRNA-1273; BNT162b2; BBIBP-CorV; COVID-19 Vaccine (Vero Cell), Inactivated; BBV152; and NVX-CoV2373 (all trade names). In a clinical setting, these vaccines are equivalent, and healthcare professionals should use whichever option is available [20]. In patients under 60 years old, Australian guidelines prefer BNT162b2, mRNA-1273, or Novavax vaccines over AZD1222 [21]. As of July 2022, ten vaccines had been approved for use in Russia. A long-acting combination monoclonal antibody (tixagevimab + cilgavimab) can be used for pre-exposure prophylaxis of COVID-19 in adults and children (aged 12 years and older weighing at least 40 kg) who are not infected with SARS-CoV-2, have not been in contact with a person infected with SARS-CoV-2, and have contraindications for vaccination against COVID-19 [22].
4.2. Use in Pregnancy
The CDC and the WHO recommend any approved COVID-19 vaccination for those pregnant, breastfeeding, trying to get pregnant now, or planning to get pregnant in the future [20]. Russian national guidelines recommend Sputnik V vaccination for pregnant women if the benefit outweighs the risk. Vaccination is contraindicated while breastfeeding [23].
4.3. Children and Adolescents
The CDC recommends the COVID-19 vaccination for anyone aged 6 months and older. BNT162b2 and mRNA-1273 are approved for use in children [19]. The WHO recommends starting the BNT162b2 vaccine at the age of five, and the mRNA-1273 vaccine at the age of twelve [24]. Vaccination is recommended for everyone over the age of five in Australia, [25] and over the age of twelve in Canada [26]. In Russia, the Gam-COVID-Vac-M vaccine is administered to children aged 12 to 17 [22].
4.4. People Who Have Had COVID-19
The CDC recommends vaccination for all people over the age of six months, regardless of whether they have a history of symptomatic or asymptomatic SARS-CoV-2 infection [19]. The WHO also supports this strategy [20]. In Australia, vaccination is recommended no sooner than three months after infection confirmation [25]. In Russia, vaccination is recommended 6 months after the disease, based on epidemic indications (including in previously vaccinated individuals) [27].
4.5. Immunocompromised Patients
For immunocompromised patients, the CDC recommends COVID-19 mRNA vaccines (BNT162b2 or mRNA-1273) for primary and booster vaccinations [19]. In this population, the WHO and Australian guidelines recommend additional doses of the BNT162b2 or mRNA-1273 vaccine [20][21]. Given the risk of more severe infection, Canadian guidelines recommend vaccinating patients with primary immunodeficiency with mRNA vaccines [28]. In Russia, the Sputnik V vaccine is recommended in this population. People with immunodeficiency who have had COVID-19 may receive Sputnik Light [27].
4.6. Booster Vaccination
This entry is adapted from the peer-reviewed paper 10.3390/bioengineering9110714
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 1 November 2022).
- Starshinova, A.; Malkova, A.; Zinchenko, U.; Kudlay, D.; Glushkova, A.; Dovgalyk, I.; Yablonskiy, P.; Shoenfeld, Y. Efficacy of Different Types of Therapy for COVID-19: A Comprehensive Review. Life 2021, 11, 753.
- Interim Statement on the Composition of Current COVID-19 Vaccines. Available online: https://www.who.int/news/item/17-06-2022-interim-statement-on--the-composition-of-current-COVID-19-vaccines (accessed on 30 August 2022).
- Mathieu, E.; Ritchie, H.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Hasell, J.; Macdonald, B.; Dattani, S.; Beltekian, D.; Ortiz-Ospina, E.; et al. Coronavirus Pandemic (COVID-19). 2020. Available online: https://ourworldindata.org/coronavirus (accessed on 30 August 2022).
- Available online: https://covid19.who.int/table (accessed on 31 August 2022).
- COVID-19 Vaccine Tracker and Landscape. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 1 November 2022).
- Kozlov, V.A.; Tikhonova, E.P.; Savchenko, A.A.; Kudryavtsev, I.V.; Andronova, N.V.; Anisimova, E.N.; Golovkin, A.S.; Demina, D.V.; Zdzitovetsky, D.E.; Kalinina, Y.U.S.; et al. Borisov. Clinical Immunology. In A Practical Guide for Infectious Disease Specialists; Polikor: Krasnoyarsk, Russia, 2021; p. 563.
- EMA. Clinical Evaluation of New Vaccines. European Medicines Agency. 2018. Available online: https://www.ema.europa.eu/en/clinical-evaluation-new-vaccines (accessed on 31 August 2022).
- Guidelines on Clinical Evaluation of Vaccines: Regulatory Expectations. Available online: https://www.who.int/publications/m/item/WHO-TRS-1004-web-annex-9 (accessed on 31 August 2022).
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211.
- Kudlay, D.; Kofiadi, I.; Khaitov, M. Peculiarities of the T Cell Immune Response in COVID-19. Vaccines 2022, 10, 242.
- The CanSino Biologics Ad5-nCoV-S COVID-19 Vaccine: What You Need to Know. Available online: https://www.who.int/news-room/feature-stories/detail/the--cansino-biologics-ad5-ncov-s--recombinant---covid-19-vaccine--what-you-need-to-know (accessed on 18 August 2022).
- Martynova, E.; Hamza, S.; Garanina, E.E.; Kabwe, E.; Markelova, M.; Shakirova, V.; Khaertynova, I.M.; Kaushal, N.; Baranwal, M.; Rizvanov, A.A.; et al. Long Term Immune Response Produced by the SputnikV Vaccine. Int. J. Mol. Sci. 2021, 22, 11211.
- Lapa, D.; Grousova, D.M.; Matusali, G.; Meschi, S.; Colavita, F.; Bettini, A.; Gramigna, G.; Francalancia, M.; Garbuglia, A.R.; Girardi, E.; et al. Retention of Neutralizing Response against SARS-CoV-2 Omicron Variant in Sputnik V-Vaccinated Individuals. Vaccines 2022, 10, 817.
- Liu, J.; Chandrashekar, A.; Sellers, D.; Barrett, J.; Lifton, M.; McMahan, K.; Sciacca, M.; VanWyk, H.; Wu, C.; Yu, J.; et al. Vaccines Elicit Highly Cross-Reactive Cellular Immunity to the SARS-CoV-2 Omicron Variant. medRxiv 2022, preprint.
- He, Q.; Mao, Q.; Zhang, J.; Bian, L.; Gao, F.; Wang, J.; Xu, M.; Liang, Z. COVID-19 Vaccines: Current Understanding on Immunogenicity, Safety, and Further Considerations. Front. Immunol. 2021, 12, 669339.
- Ashmawy, R.; Hamdy, N.A.; Elhadi, Y.A.M.; Alqutub, S.T.; Esmail, O.F.; Abdou, M.S.M.; Reyad, O.A.; El-Ganainy, S.O.; Gad, B.K.; El-Deen, A.E.-S.N.; et al. A Meta-Analysis on the Safety and Immunogenicity of COVID-19 Vaccines. J. Prim. Care Community Health 2022, 13, 21501319221089256.
- Guo, W.; Deguise, J.; Tian, Y.; Huang, P.C.E.; Goru, R.; Yang, Q.; Peng, S.; Zhang, L.; Zhao, L.; Xie, J.; et al. Profiling COVID-19 Vaccine Adverse Events by Statistical and Ontological Analysis of VAERS Case Reports. Front. Pharmacol. 2022, 13, 870599.
- Clinical Guidance for COVID-19 Vaccination|CDC. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html (accessed on 31 August 2022).
- Coronavirus Disease (COVID-19): Vaccines. Available online: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-vaccines (accessed on 31 August 2022).
- Care AGD of H and A. ATAGI Clinical Guidance for COVID-19 Vaccine Providers. Australian Government Department of Health and Aged Care; 2021. Available online: https://www.health.gov.au/initiatives-and-programs/covid-19-vaccines/advice-for-providers/clinical-guidance (accessed on 31 August 2022).
- Temporary Guidelines for Prevention, Diagnosis and Treatment of Novel Coronavirus Disease (COVID-19) Version 16.pdf. Available online: https://static-0.minzdrav.gov.ru/system/attachments/attaches/000/060/193/original/%D0%92%D0%9C%D0%A0_COVID-19_V16.pdf (accessed on 30 August 2022).
- 05072021_MR_Preg_v4.pdf. Available online: https://static-0.minzdrav.gov.ru/system/attachments/attaches/000/057/333/original/05072021_MR_Preg_v4.pdf (accessed on 20 July 2022).
- Vaxart. A Phase 2, Double-Blind, Multi-Center, Randomized, Placebo-Controlled, Dose-Ranging Trial to Determine the Safety, Immunogenicity and Efficacy of an Adenoviral-Vector Based Vaccine Expressing Severe Acute Respiratory Syndrome (SARS-CoV-2) and dsRNA Adjuvant Administered Orally. clinicaltrials.gov; Report No.: NCT05067933. May 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05067933 (accessed on 14 August 2022).
- Vaxart COVID-19 Oral Vaccine. Available online: https://www.precisionvaccinations.com/vaccines/vaxart-covid-19-oral-vaccine (accessed on 15 August 2022).
- Public Health Agency of Canada. Archive 38: COVID-19 Vaccine Guidance Updates: Canadian Immunization Guide: June 21 2022. Available online: https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/summary-updates-canadian-immunization-guide-june-21-2022-covid-19-vaccines.html (accessed on 31 August 2022).
- Temporary Guidelines. The Procedure for Vaccination against a New Coronavirus Infection (COVID-19). 2022. Available online: https://static-0.minzdrav.gov.ru/system/attachments/attaches/000/060/087/original/%D0%9C%D0%B5%D1%82%D0%BE%D0%B4%D0%B8%D1%87%D0%B5%D1%81%D0%BA%D0%B8%D0%B5_%D1%80%D0%B5%D0%BA%D0%BE%D0%BC%D0%B5%D0%BD%D0%B4%D0%B0%D1%86%D0%B8%D0%B8_02062022_%282%29.pdf?1655803717 (accessed on 18 August 2022).
- Public Health Agency of Canada. COVID-19 Vaccine: Canadian Immunization Guide. 2021. Available online: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-26-covid-19-vaccine.html (accessed on 31 August 2022).
- Letter of the Ministry of Health of Russia. Dated December 22 2021 N 30-4/I/2-21694. On the Submission of Updated Temporary Guidelines. The Procedure for Vaccination against a New Coronavirus Infection (COVID-19). Available online: https://spboms.ru/sites/default/files/pismo_minzdrava_rossii_ot_22_12_2021_n_30-4_i_2-21694_o.pdf (accessed on 31 August 2022).
- COVID-19 Vaccination: Booster Dose Resources—GOV.UK. Available online: https://www.gov.uk/government/publications/covid-19-vaccination-booster-dose-resources (accessed on 31 August 2022).
- About Clarification of the Procedure for Re-Vaccination against COVID-19. Available online: http://www.7gsp.by/polezno-znat/stati/558-o-razyasnenii-poryadka-provedeniya-povtornoj-vaktsinatsii-protiv-covid-19 (accessed on 14 August 2022).
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546.
- Abu-Raddad, L.J.; Chemaitelly, H.; Ayoub, H.H.; AlMukdad, S.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Tang, P.; Hasan, M.R.; Coyle, P.; et al. Effect of mRNA Vaccine Boosters against SARS-CoV-2 Omicron Infection in Qatar. N. Engl. J. Med. 2022, 386, 1804–1816.
- CDC. Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention; 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html (accessed on 31 August 2022).
- UK Grants World’s First Approval for Moderna’s Omicron Covid Vaccine. Bloomberg.com. 2022. Available online: https://www.bloomberg.com/news/articles/2022-08-15/uk-takes-world-s-first-step-toward-omicron-tailored-covid-shot (accessed on 25 August 2022).
- Au, W.Y.; Cheung, P.P.H. Effectiveness of heterologous and homologous COVID-19 vaccine regimens: Living systematic review with network meta-analysis. BMJ 2022, 377, e069989.
