Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Medicine, Research & Experimental
Trace amines are a chemical group of amines and their isomers, included in the biogenic amines’ family. They are natural compounds with low molecular weight, formed during the natural metabolism of animals, plants, and microorganisms from amino acids precursors.
- trace amines
- neuromodulation
- neurotransmitters
- TAARs
1. Introduction
Trace amines are a class of natural endogenous amines with low molecular weight, formed during the natural metabolism of animals, plants, and microorganisms from amino acids precursors [1]. In general, the trace amine family includes all endogenous amines present at a low nanomolar concentrations in invertebrate and vertebrate systems, like m- and p-tyramine, β-phenylethylamine, p-octopamine, tryptamine, p-synephrine and N,N-dimethyltryptamine [2]. In invertebrates, they act directly as neurotransmitters [3], while in mammalians their roles were mainly attributed to that of supporting catecholamine actions. Not by chance, trace amines are in different areas of mammalian brain and their distribution is strictly linked to the projection domains of noradrenaline, dopamine and serotonin pathways [2]. However, at present they are considered to play important roles in both neurotransmission and neuromodulation. Moreover, ingestion of high amounts of trace amines can lead to toxicity, including indirect sympathomimetic effects resulting in amphetamine-like responses [2][4], which will be discussed next.
Trace amines are obtained through direct and selective action of decarboxylases on amino acids by removing the carboxyl group. In β-phenylethylamine, tyramine and tryptamine synthesis, the aromatic L- amino acid decarboxylase (AADC) mediates L-phenylalanine, L-tyrosine, and L-tryptophan decarboxylation, whereas octopamine is formed after hydroxylation of p-tyramine by dopamine β–decarboxylase. In addition, N-methylation represents another pathway by which tryptamine can be metabolized, being that the formation of N,N-dimethyltryptamine is catalyzed by indolethylamine–N–methyltransferase [5].
2. Trace Amines
2.1. Sources
Trace amines are naturally present in the human organism, even if their endogenous production does not represent their primary source [6]. In fact, fermented cheese, fermented cabbage, chocolate, raw and smoked meat, raw and fish products, alcoholic beverages, soybeans products are some of the foodstuffs where trace amines have been detected. Their presence and amounts in food varies, which can be affected by intrinsic and extrinsic factors, such as: availability of free amino acids; presence of microorganism with aminogenic capacity, namely decarboxylase-positive microorganisms, and overall conditions that allow microorganisms’ growth [7].
Moreover, food matrixes own different profiles of trace amines, depending on free amino acid and protein amounts in the starting materials [8]. They are usually present in foodstuffs following degradation of proteins and peptides, including proteolysis by microorganisms. Biogenic amines and consequentially trace amines’ production occurs in the presence of microorganisms able to decarboxylate amino acids through substrate-specific enzymes, as stated [9][10]. Decarboxylases are a group of enzymes that belong to the pyridoxal-phosphate-dependent family, where pyridoxal-5′-phosphate represents the coenzyme. Decarboxylase-positive microorganisms are naturally present in food, being often added during the manufacturing processes. Thus, trace amines production depends on the microorganism strain and its’ habitat conditions [11]. Not by chance, differences in the quantitative and qualitative profiles of trace amines have been observed concurrently with microbiological changes, as a result of the variable response of the decarboxylase activity to environmental factors [12]. According to Gardini et al., temperature, pH and salt concentration represent the most relevant factors affecting biogenic amines quantity in foods, but storage and production processes may also modulate their profile. These conditions act synergically augmenting or reducing the presence of trace amines. Tyramine, and other biogenic amines, are usually predominant during ripening, even if a decrease in their maximum concentration occurs in conformity with variations in the microbiota [13]. Several amines, such as histamine, tyramine and phenylethylamine have been found in different types of ripened cheese, where they are mainly produced and accumulated by lactic acid bacteria or by microorganisms with decarboxylase activity (e.g., Bacillus, Pseudomonas, Escherichia, Enterobacter, Salmonella, Shigella, Staphylococcus, Streptococcus, Lactobacillus, Enterococcus, Lactococcus, and Leuconostoc) [6][12]. Even so, many elements promote or inhibit microbial activity during spontaneous fermentation: the use of food additives, or other synthetic and natural compounds can in fact inhibit the formation of amines [14]. Caraway and onion reduce the amount of the biogenic amine in sauerkraut, depending also on the temperature during its preparation, for instance. The salt concentration is another impacting factor that contributes to a reduction in trace amines in foods, as the activity of decarboxylating microbiota is inhibited by the addition of salt depending on bacteria species present [12]. The following table shows some sources and amounts of trace amines in food (Table 1).
Table 1. Major trace amines tyramine (TYR), β-phenylethylamine (PEA), and tryptamine (TRYP) found in food.
| Sample | Trace Amines (mg/kg) | Reference | ||
|---|---|---|---|---|
| TYR | PEA | TRYP | ||
| Soppresata | 8.12–511.44 | 10.56–19.90 | ND | [15] |
| Sausages | 10.33–338.85 | ND | ND | [15] |
| Salami | 77.14 | 3.2 | 1.63 | [16] |
| Hamburger | 1.5–27.0 | ND | 9.3–13.5 | [17] |
| Chocolate | 3.11 | 2.67 | 1.43 | [16] |
| Coffee | 1.26–16.41 | / | / | [18][19] |
| Fermented cabbage | 60.66 | 0.73 | 0.18 | [16] |
| Azeitão cheese 30 days of ripening |
122 | / | ≅ 65 | [13] |
| Parmigiano cheese | 3.75 | 0.2 | 0.07 | [16] |
| Yogurt | ND | 0.001 | ND | [16] |
| Leerdamer | ND | 0.005 | 0.04 | [16] |
| Red wine | 1.93 | 0.61 | 0.03 | [16] |
| Red wine Abruzzo (Chieti, Italy) |
11.94 ± 7.79 mg L−1, mean ± s.d | ND | / | [20] |
| Rosé wine Abruzzo (Chieti, Italy) |
0.27 ± 0.23 mg L−1, mean ± s.d. | 0.16 | / | [20] |
| White wine Abruzzo (Chieti, Italy) |
ND | ND | / | [20] |
| Fiore Sardo Sheep Cheese (Sardinia, Italy) |
350 ± 300 mg/kg, mean ± s.d | / | / | [21] |
| Lupin Luteus Spontaneously fermented |
32.6 | 128.8 | / | [22] |
| Lupin Albus Spontaneously fermented |
69.1 | 144.1 | / | [22] |
| Soybean Rudoji Spontaneously fermented |
30.1 ± 3.1 | 230.1 ± 8.6 | / | [22] |
| Soybean Progress Spontaneously fermented |
27.8 ± 3.2 | 234.5 ± 12.3 | / | [22] |
| Feta cheese | 0–246 | 0.77–4.94 | 2.18–6.24 | [23] |
ND: not detected; /: no information.
2.2. Pharmacology
In mammals, the pharmacological proprieties of trace amines are traditionally attributed to that of support classical biogenic amine activity. Nevertheless, they own specific receptors, Trace Amine—Associated Receptors (TAARs), which are G protein-coupled receptors (GPCR). TAARs own distinctive structural characteristics of the rhodopsin-beta adrenergic receptor superfamily with a chain formed by 7 transmembrane domains [24][25]. At the start of 2001, the TAARs family was discovered through two independent works. Their genes were found in several species, namely humans, rats, mice, and chimpanzees, while interspecies differences in the distribution of these receptors were also found [26][27][28]. After that, their roles have been further investigated, highlighting their importance in both invertebrates and vertebrates. Only six of have been reported so far in humans. Moreover, when taking TAAR2, TAAR5, TAAR6, TAAR8 or TAAR9, only TAAR1 demonstrates a high affinity towards trace amines and will be the only one approached next.
2.2.1. TAAR1 and Its Agonists
TAAR1 receptor is the oldest known member of the TAAR family, and it has been shown to have interactions especially with β-phenylethylamine, but also with tyramine, tryptamine, octopamine, and the dopamine metabolite, 3-methoxytyramine [5]. In addition, a thyroid hormone derivative, 3-thyronamine (T1AM), and isoproterenol activate TAAR1 too [29]. Moreover, psychotropic agents, such as 3,4-methylenedioxymethamphetamine (MDMA), lysergic acid, amphetamine and methamphetamine are partial agonists to this receptor [4][30][31][32].
Current understanding confirms an interplay between the TAAR1 and the dopamine systems. β-phenylethylamine and tyramine are synthesized in dopaminergic terminals, but are not stored within synaptic vesicles. Thus, they can easily diffuse across membranes. On the other hand, their reuptake into presynaptic terminals has no specific transporter and seems to occur via the organic cation transporter 2 (OCT2) [5]. Not by chance, the TAARs family is also expressed in various brain areas, including olfactory epithelium neurons, where it may act as a new class of receptors for different volatile compounds [5]. Moreover, these receptors are present in the crucial brain areas linked to the monoaminergic system, e.g., those implicated in mood regulation, emotion, learning, cognition, attention, and reward [2]. Altered levels of monoamines seem to be involved in various neuropsychiatric disorders, including schizophrenia, depression, attention deficit hyperactivity disorder (ADHD), bipolar disorder and anxiety. Actually, amphetamines cause an increase in the extracellular levels of dopamine in the brain, and the discovery that amphetamine-like compounds act on TAAR1 can make this receptor as a possible therapeutic target for their side effects [32]. Furthermore, in vitro studies have revealed a possible relationship between TAAR1, monoamine autoreceptors and monoamine transporters [32].
2.2.2. TAAR1 Localization
TAAR1 is primarily located intracellularly both in the Central Nervous System (CNS) and in the periphery, and in neurons and glial cells. Anatomically, considerable overlaps exist between the expression of TAAR1 and dopaminergic and serotonergic brain structures [33]. TAAR1 is also present in the periphery, namely on leukocytes [33], and in the vasculature [5], as well as in the stomach, intestine, kidneys, liver, and pancreas [5]. In addition, TAAR1 appears to regulate hormone release in response to different stimuli and nutrients, assuming thus a role as a novel target for metabolic diseases [34]. Due to its presence on leukocytes, TAAR1 may be a target in inflammatory bowel disease, even if other TAARs seem to be able to activate leukocytes recruitment to the gastrointestinal mucosa and act through other mechanism involved in the outset of that intestinal disease [34][35].
Another important feature of TAAR1 is its predominantly intracellular location. TAAR1 causes both pre- and postsynaptic effects. Though it is considered a GPCR receptor, and being associated with the cellular membrane, the exact place of TAAR1 in the cell is not yet known, and there is still a great need for research in this area [5]. Once ligands bind to the TAAR1 pocket region, different signaling pathways are activated. TAAR1 can couple to Gs and Gq. Hence, upon stimulation, it triggers the accumulation of intracellular adenosine 3′,5′-monophosphate (cAMP) via adenylyl cyclase or phosphoinositol metabolism. Subsequently, protein kinases B and C are activated, promoting extracellular Ca2+ influx. Excessive Ca2+ influx may lead to oxidative stress acting on the endoplasmatic reticulum and generating mitochondrial dysfunction. TAARs can also operate through β–arrestin 2, a protein associated with their desensitization [36]. Once activated, GPCR regulates various effectors through its subunits (Gα and Gβ) and following their phosphorylation through the action of kinase enzymes, β–arrestin 2 can be recruited. On the other hand, TAAR1 can translocate to the cell surface after heterodimerization with dopamine 2 (D2) receptors, an effect promoted by the Gi signal transduction cascade [5][37], and also involvement of the β–arrestin 2 pathway [37]. In addition, TAAR1 ligands can activate G protein-coupled inwardly rectifying potassium channels (GIRK), which is a family of potassium channels located mostly in the brain, but also in the heart (Figure 1). Once ligands stimulate TAAR1, the heterodimer Gβγ, formed from the GPCR, activates GIRK causing hyperpolarization of the membrane [38]. In summary, when activated, TAAR1 leads to an increase in intracellular cAMP production with protein kinase A and protein kinase C activation and phosphorylation of downstream targets, although other mechanisms can be involved. In fact, it also activates a G protein-independent, β-arrestin 2-dependent pathway involving protein kinase B (AKT)/glycogen synthase kinase-3 (GSK-3). Moreover, it has become evident that TAAR 1 also interacts with dopamine transporters (DAT) and dopamine D2 receptors [39][40][41]. Nevertheless, phosphorylation of the DAT through TAAR1-stimulated activation can lead to DAT internalization, which leads to reduced dopamine uptake under certain conditions [32] (Figure 1).
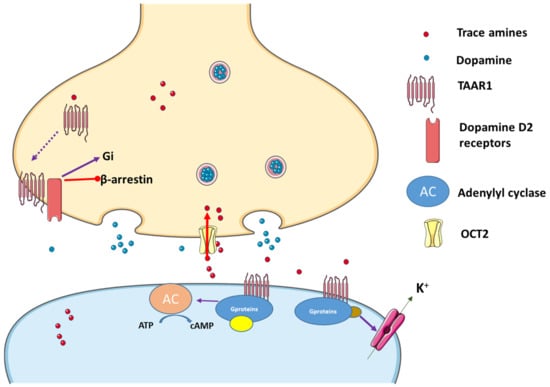
Figure 1. Trace amines activate TAAR1, leading to adenylyl cyclase (AC) activation and downstream activation of protein kinase A and protein kinase C. Interactions with the dopaminergic system can also occur and TAAR1 may translocate to the cell surface after heterodimerization with dopamine receptor 2 (D2), an effect that promotes preferential signaling related to dopamine and through the Gi signal transduction cascade, while blocking the β-arrestin 2 pathway. In addition, K+ channels can be activated in the presence of TAAR agonists. Figure built resourcing to the blocks of Servier Medical Art. Figure taken from [1].
2.2.3. Trace Amines as Indirect Sympathomimetic Agents
Before the discovery of the TAARs family, trace amines were mainly considered indirect sympathomimetic agents, causing vasoconstriction, and consequentially increasing blood pressure in vascular beds. It is well-known that a high concentration of trace amines, especially tyramine and β-phenylethylamine, mimics the action of amphetamines. They enter the pre-synaptic terminal neurons causing catecholamine displacement from cytoplasmic pools. Consequently, tyramine and β-phenylethylamine lead to the release of noradrenaline in the synaptic cleft, which causes vasoconstriction by stimulating α-adrenoceptors [4]. In fact, an increase in systolic blood pressure, following a high ingestion of tyramine, is known as the “tyramine pressor response” [42]. Some results suggest that the indirect sympathomimetic effects played by trace amines are not their only mechanisms of action in the vascular system, since they can interact with other receptors [43]. Evidence has been shown that trace amines, particularly tyramine, β-phenylethylamine and octopamine, act directly on α1- adrenoreceptors, forming different responses based on the vessel evaluated. Overlooking the indirect sympathomimetic effect produced by trace amines, the α1-adrenoceptors activation causes intensive vasoconstriction, suggesting a direct interaction between trace amines and the receptor. Tyramine and β-phenylethylamine are also able to promote a contractive response in guinea pig and rat isolated gut preparations, quite the opposite to what occurs in the presence of sympathomimetic amines [44]. Research carried out using a high concentration of an antagonist of TAAR1, called EPPTB (RO-5212773), has shown a reduction in the vasocontractile response in the porcine coronary artery [43]. Additionally, tyramine and β-phenylethylamine exhibit a partial antagonism towards β1 and β2 adrenoceptors, while octopamine has shown a partial β1 agonism and a partial orthosteric antagonist in β2 adrenoceptors [29]. The inhibitory effects seem to depend on the doses used and the animal species under evaluation. These data highlight that further research is needed to understand completely the mechanisms of action of trace amines on the TAARs family.
2.2.4. Trace Amines Pharmacokinetics
Regarding their pharmacokinetics, trace amines show a rapid turnover due to their metabolization to biologically inactive products mainly through monoamino oxidase (MAO). MAOs encompass two different intracellular isoenzymes located in mitochondria, with various substrates and specific organ locations. MAO-A is primary found in the stomach, in the intestine and the gut, in addition to the placenta and sympathetic neurons. MAO-B is predominant in the brain and in the serotoninergic neurons, where β-phenylethylamine represents its selective substrate [5]. Differently from β-phenylethylamine, other compounds do not show a pronounced selectivity towards either MAO-A or MAO-B. For patients with MAOs deficiencies or using MAO inhibitors (MAOI) as drug therapy, an increase of trace amines could contribute to the development of toxic responses [45], as it will be discussed next.
2.3. Toxic Effects of Trace Amines
Though trace amines are found in nanomolar concentrations in the human organism, their toxicology aspects are being debated until now and are even controversial. According to the European Food Safety Authority (EFSA) Panel on Biological Hazard (BIOHAZ), tyramine and histamine are the most common and toxic biogenic amines present in foods, although their risk assessment is still not quantified [45]. Nevertheless, some studies have advocated for their toxicity.
Historically, the first report of trace amines toxicity was associated with hypertension. A woman taking MAOI suffered strong headaches after eating cheese, being these effects thereafter known as “cheese syndrome” [5][46]. Generally, trace amines ingested in the diet are immediately detoxified by MAOs, but in people with dysfunctional MAO or inhibited MAO, they can lead to serious toxicity, mainly in the vascular system [12], as explained. Tyramine, β-phenylethylamine and tryptamine are the trace amines with the highest risk for these acute severe effects. They may generate vasoconstriction responses including headaches and even hypertensive crises, but also vomiting, nausea, pupil dilatation, and breathing difficulties [45]. According to different data reported in BIOHAZ review about biogenic amines, the food-drug interaction is one of the most common causes of toxicity related to trace amines, especially in the case of tyramine. The threshold for tyramine for healthy individuals is established at 600 mg. Above that concentration, tyramine could provoke an increase of almost 30 mmHg in the systolic blood pressure. Conversely, clinical cases regarding intoxications after trace amines are not very frequent in the literature. Most common toxidromes regarding trace amines are related to pharmacokinetic interactions with cheese or other foods in patients who were treated with MAOI drugs. However, IMAOs are not so frequently used nowadays in depression therapy. One case report, nonetheless, describes a 34-year-old woman, with a depressive state and being treated with phenelzine, a non-selective and irreversible MAOI. This woman suffered a myocardial infarct resulting from a MAOI-induced hypertensive crisis, after eating cheese [47]. EFSA also reported data regarding toxicity of trace amines in patients in treatment under Reversible Inhibitors of Monoamine Oxidase (RIMAs), a new generation of monoamine oxidase inhibitors. In these cases, the threshold is higher for a safe tyramine use [45].
Another toxicological effect played by trace amines is migraine. An increase in tyramine and β-phenylethylamine causes cerebral vasoconstriction and dilatation, causing pain. Usually, the migraine is supported by nausea being seen in susceptible patients after the ingestion of foods rich of tyramine and β-phenylethylamine. Those effects usually begin 1-12 h after ingestion. Their effects are intensified by the consumption of ethanol or coffee, due to the amplification in vascular responses [48]. In addition, ethanol can inhibit MAO too and augments the membrane permeability of the gut, thus increasing trace amines absorption without major metabolization [12]. Tyramine and β-phenylethylamine have also been found in tobacco. Combustion of cigarettes releases compounds that inhibit MAO, contributing to an increase in trace amines and consequentially noradrenaline concentrations by the indirect mechanisms. Furthermore, in smokers, levels of MAO-B and MAO-A are decreased almost by 40%, which can impair metabolism, and potentially lead to interactions but other studies are required [4].
Finally, synephrine is found to have a different toxicological profile regarding trace amines as stated in a recent review [49]. In conclusion, though MAOIs have a good profile in safety terms, and technological factors affecting trace amines content in foods are being studied, patients need to limit the intake of foods particularly rich in tyramine to avoid toxicity [12][1], especially if taking drugs affecting their metabolism or if they have high risk of vasoconstriction complications.
This entry is adapted from the peer-reviewed paper 10.3390/biom12121793
References
- Costa, V.M.; Grando, L.G.R.; Milandri, E.; Nardi, J.; Teixeira, P.; Mladěnka, P.; Remião, F.; on behalf of The OEMONOM.; Natural Sympathomimetic Drugs: From Pharmacology to Toxicology. Biomolecules 2022, 12, 1793, .
- Burchett, S.A.; Hicks, T.P. The mysterious trace amines: Protean neuromodulators of synaptic transmission in mammalian brain. Prog. Neurobiol. 2006, 79, 223–246.
- Roeder, T. Chapter 1—Trace Amines: An Overview. In Trace Amines and Neurological Disorders; Farooqui, T., Farooqui, A.A., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 3–9.
- Broadley, K.J. The vascular effects of trace amines and amphetamines. Pharmacol. Ther. 2010, 125, 363–375.
- Gainetdinov, R.R.; Hoener, M.C.; Berry, M.D. Trace Amines and Their Receptors. Pharmacol. Rev. 2018, 70, 549–620.
- Ruiz-Capillas, C.; Herrero, A.M. Impact of Biogenic Amines on Food Quality and Safety. Foods 2019, 8, 62.
- Ten, B.B.; Damink, C.; Joosten, H.M.L.J.; Huis in ’t Veld, J.H.J. Occurrence and formation of biologically active amines in foods. Int. J. Food Microbiol. 1990, 11, 73–84.
- Doeun, D.; Davaatseren, M.; Chung, M.S. Biogenic amines in foods. Food Sci. Biotechnol. 2017, 26, 1463–1474.
- Zucchi, R.; Chiellini, G.; Scanlan, T.S.; Grandy, D.K. Trace amine-associated receptors and their ligands. Br. J. Pharmacol. 2006, 149, 967–978.
- Stratton, J.E.; Hutkins, R.W.; Taylor, S.L. Biogenic Amines in Cheese and other Fermented Foods: A Review. J. Food Prot. 1991, 54, 460–470.
- Marcobal, A.; de las Rivas, B.; Landete, J.M.; Tabera, L.; Muñoz, R. Tyramine and Phenylethylamine Biosynthesis by Food Bacteria. Crit. Rev. Food Sci. Nutr. 2012, 52, 448–467.
- Gardini, F.; Özogul, Y.; Suzzi, G.; Tabanelli, G.; Özogul, F. Technological Factors Affecting Biogenic Amine Content in Foods: A Review. Front. Microbiol. 2016, 7, 1218.
- Ferreira, I.M.; Pinho, O. Biogenic amines in Portuguese traditional foods and wines. J. Food Prot. 2006, 69, 2293–2303.
- Majcherczyk, J.; Surówka, K. Effects of onion or caraway on the formation of biogenic amines during sauerkraut fermentation and refrigerated storage. Food Chem. 2019, 298, 125083.
- Parente, E.; Martuscelli, M.; Gardini, F.; Grieco, S.; Crudele, M.A.; Suzzi, G. Evolution of microbial populations and biogenic amine production in dry sausages produced in Southern Italy. J. Appl. Microbiol. 2001, 90, 882–891.
- Mayr, C.M.; Schieberle, P. Development of stable isotope dilution assays for the simultaneous quantitation of biogenic amines and polyamines in foods by LC-MS/MS. J. Agric. Food Chem. 2012, 60, 3026–3032.
- Durlu-Özkaya, F.; Ayhan, K.; Vural, N. Biogenic amines produced by Enterobacteriaceae isolated from meat products. Meat Sci. 2001, 58, 163–166.
- Andersen, G.; Marcinek, P.; Sulzinger, N.; Schieberle, P.; Krautwurst, D. Food sources and biomolecular targets of tyramine. Nutr. Rev. 2019, 77, 107–115.
- Restuccia, D.; Spizzirri, U.G.; Parisi, O.I.; Cirillo, G.; Picci, N. Brewing effect on levels of biogenic amines in different coffee samples as determined by LC-UV. Food Chem. 2015, 175, 143–150.
- Martuscelli, M.; Arfelli, G.; Manetta, A.C.; Suzzi, G. Biogenic amines content as a measure of the quality of wines of Abruzzo (Italy). Food Chem. 2013, 140, 590–597.
- Zazzu, C.; Addis, M.; Caredda, M.; Scintu, M.F.; Piredda, G.; Sanna, G. Biogenic Amines in Traditional Fiore Sardo PDO Sheep Cheese: Assessment, Validation and Application of an RP-HPLC-DAD-UV Method. Separations 2019, 6, 11.
- Bartkiene, E.; Krungleviciute, V.; Juodeikiene, G.; Vidmantiene, D.; Maknickiene, Z. Solid state fermentation with lactic acid bacteria to improve the nutritional quality of lupin and soya bean. J. Sci. Food Agric. 2015, 95, 1336–1342.
- Valsamaki, K.; Michaelidou, A.; Polychroniadou, A. Biogenic amine production in Feta cheese. Food Chem. 2000, 71, 259–266.
- Lindemann, L.; Meyer, C.A.; Jeanneau, K.; Bradaia, A.; Ozmen, L.; Bluethmann, H.; Bettler, B.; Wettstein, J.G.; Borroni, E.; Moreau, J.L.; et al. Trace amine-associated receptor 1 modulates dopaminergic activity. J. Pharmacol. Exp. Ther. 2008, 324, 948–956.
- Khan, M.Z.; Nawaz, W. The emerging roles of human trace amines and human trace amine-associated receptors (hTAARs) in central nervous system. Biomed. Pharmacother. 2016, 83, 439–449.
- Narang, D.; Tomlinson, S.; Holt, A.; Mousseau, D.D.; Baker, G.B. Trace Amines and Their Relevance to Psychiatry and Neurology: A Brief Overview. Bull. Clin. Psychopharmacol. 2011, 21, 73–79.
- Borowsky, B.; Adham, N.; Jones, K.A.; Raddatz, R.; Artymyshyn, R.; Ogozalek, K.L.; Durkin, M.M.; Lakhlani, P.P.; Bonini, J.A.; Pathirana, S.; et al. Trace amines: Identification of a family of mammalian G protein-coupled receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 8966–8971.
- Bunzow, J.R.; Sonders, M.S.; Arttamangkul, S.; Harrison, L.M.; Zhang, G.; Quigley, D.I.; Darland, T.; Suchland, K.L.; Pasumamula, S.; Kennedy, J.L.; et al. Amphetamine, 3,4-Methylenedioxymethamphetamine, Lysergic Acid Diethylamide, and Metabolites of the Catecholamine Neurotransmitters Are Agonists of a Rat Trace Amine Receptor. Mol. Pharmacol. 2001, 60, 1181–1188.
- Kleinau, G.; Pratzka, J.; Nürnberg, D.; Grüters, A.; Führer-Sakel, D.; Krude, H.; Köhrle, J.; Schöneberg, T.; Biebermann, H. Differential modulation of Beta-adrenergic receptor signaling by trace amine-associated receptor 1 agonists. PLoS ONE 2011, 6, e27073.
- Frascarelli, S.; Ghelardoni, S.; Chiellini, G.; Vargiu, R.; Ronca-Testoni, S.; Scanlan, T.S.; Grandy, D.K.; Zucchi, R. Cardiac effects of trace amines: Pharmacological characterization of trace amine-associated receptors. Eur. J. Pharmacol. 2008, 587, 231–236.
- Liu, J.; Wu, R.; Li, J.X. TAAR1 and Psychostimulant Addiction. Cell. Mol. Neurobiol. 2020, 40, 229–238.
- Pei, Y.; Asif-Malik, A.; Canales, J.J. Trace Amines and the Trace Amine-Associated Receptor 1: Pharmacology, Neurochemistry, and Clinical Implications. Front. Neurosci. 2016, 10, 148.
- Babusyte, A.; Kotthoff, M.; Fiedler, J.; Krautwurst, D. Biogenic amines activate blood leukocytes via trace amine-associated receptors TAAR1 and TAAR2. J. Leukoc. Biol. 2013, 93, 387–394.
- Christian, S.L.; Berry, M.D. Trace Amine-Associated Receptors as Novel Therapeutic Targets for Immunomodulatory Disorders. Front. Pharmacol. 2018, 9, 680.
- Vitale, S.; Strisciuglio, C.; Pisapia, L.; Miele, E.; Barba, P.; Vitale, A.; Cenni, S.; Bassi, V.; Maglio, M.; Del Pozzo, G.; et al. Cytokine production profile in intestinal mucosa of paediatric inflammatory bowel disease. PLoS ONE 2017, 12, e0182313.
- Latapy, C.; Beaulieu, J.M. β-Arrestins in the central nervous system. Prog. Mol. Biol. Transl. Sci. 2013, 118, 267–295.
- Espinoza, S.; Masri, B.; Salahpour, A.; Gainetdinov, R.R. BRET approaches to characterize dopamine and TAAR1 receptor pharmacology and signaling. Methods Mol. Biol. 2013, 964, 107–122.
- Kano, H.; Toyama, Y.; Imai, S.; Iwahashi, Y.; Mase, Y.; Yokogawa, M.; Osawa, M.; Shimada, I. Structural mechanism underlying G protein family-specific regulation of G protein-gated inwardly rectifying potassium channel. Nat. Commun. 2019, 10, 2008.
- Xie, Z.; Miller, G.M. Trace Amine-Associated Receptor 1 Is a Modulator of the Dopamine Transporter. J. Pharmacol. Exp. Ther. 2007, 321, 128.
- Xie, Z.; Westmoreland, S.V.; Bahn, M.E.; Chen, G.-L.; Yang, H.; Vallender, E.J.; Yao, W.-D.; Madras, B.K.; Miller, G.M. Rhesus Monkey Trace Amine-Associated Receptor 1 Signaling: Enhancement by Monoamine Transporters and Attenuation by the D2 Autoreceptor in Vitro. J. Pharmacol. Exp. Ther. 2007, 321, 116.
- Grandy, D.K. Trace amine-associated receptor 1-Family archetype or iconoclast? Pharmacol. Ther. 2007, 116, 355–390.
- Herbert, A.A.; Kidd, E.J.; Broadley, K.J. Dietary trace amine-dependent vasoconstriction in porcine coronary artery. Br. J. Pharmacol. 2008, 155, 525–534.
- Koh, A.H.W.; Chess-Williams, R.; Lohning, A.E. Differential mechanisms of action of the trace amines octopamine, synephrine and tyramine on the porcine coronary and mesenteric artery. Sci. Rep. 2019, 9, 10925.
- Broadley, K.J.; Akhtar Anwar, M.; Herbert, A.A.; Fehler, M.; Jones, E.M.; Davies, W.E.; Kidd, E.J.; Ford, W.R. Effects of dietary amines on the gut and its vasculature. Br. J. Nutr. 2009, 101, 1645–1652.
- Collins, J.D.; Noerrung, B.; Budka, H.; Andreoletti, O.; Buncic, S.; Griffin, J.; Hald, T.; Havelaar, A.; Hope, J.; Klein, G.; et al. Scientific Opinion on risk based control of biogenic amine formation in fermented foods. EFSA J. 2011, 9, 2393.
- Victor, L.; Marina, C.-E.; Maria, F.; Miguel, A.A. Toxicological Effects of Dietary Biogenic Amines. Curr. Nutr. Food Sci. 2010, 6, 145–156.
- Ngo, A.S.; Ho, R.Y.; Olson, K.R. Phenelzine-induced myocardial injury: A case report. J. Med. Toxicol. 2010, 6, 431–434.
- Costa, M.R.; Glória, M.B.A. Migraine and Diet. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 3940–3947.
- Gillman, P.K. Monoamine Oxidase Inhibitors: A Review Concerning Dietary Tyramine and Drug Interactions. PsychoTrop. Comment. 2016, 16, 1–90.
This entry is offline, you can click here to edit this entry!
