Chitooligosaccharides, also known as chitosan oligomers or chitooligomers, are made up of chitosan with a degree of polymerization (DP) that is less than 20 and an average molecular weight (MW) that is lower than 3.9 kDa. COS can be produced through enzymatic conversions using chitinases, physical and chemical applications, or a combination of these strategies. COS is of significant interest for pharmacological and medical applications due to its increased water solubility and non-toxicity, with a wide range of bioactivities, including antibacterial, anti-inflammatory, anti-obesity, neuroprotective, anticancer, and antioxidant effects.
1. Introduction
Chitin, a mucopolysaccharide, is produced by many living organisms and is usually present in a complex with other polysaccharides and proteins in insects, crustaceans, arachnids, myriapods, nematodes, algae, and fungi [
1]. Chitin is a linear polysaccharide composed of (1 → 4) linked 2-acetamido-2-deoxy-
β-d-glucopyranosyl units and occurs naturally in three polymorphic forms with different orientations of the microfibrils, known as
α-,
β-, and
γ-chitin [
2]. Chitin has been the focus of numerous therapeutic uses in addition to serving as a precursor for producing chitosan and chitooligosaccharides. Chitin has several uses in food, agriculture, wastewater treatment, textiles, microbiology, nanotechnology, chemistry, and material science [
3]. It is biodegradable and is also considered as a promising biomaterial for tissue engineering and stem cell technologies [
4].
Chitosan [poly-(
β-1/4)-2-amino-2-deoxy-D-glucopyranose] is a natural nontoxic linear polysaccharide biopolymer produced through the deacetylation of chitin [
5]. Commercial chitosan is made by deacetylating naturally occurring chitin and is used in dietary supplements, organic fertilizers, and cosmetics [
6,
7]. Chitin and chitosan can be distinguished based on the degree of acetylation of the D-glucosamine units. Chitin includes over 70% acetylated units, whereas chitosan contains less than 30% acetylation. In the presence of organic acids, including formic acid, acetic acid, and ascorbic acid, chitosan forms salt and becomes water soluble [
8]. Chitosan possesses three reactive functional groups, including an amino- or N-acetamide group and two primary and secondary hydroxyl groups at the C-2, C-3, and C-6 positions. The amino- or N-acetamide groups distinguish the structure and physicochemical properties of various chitosans [
9]. The fraction of N-acetylated residues (FA), degree of polymerization (DP), molecular weight (MW), MW distribution, and pattern or sequence of N-acetylation (PA) can be used to classify chitosan [
10].
Chitosans with a DP of less than 20 and an average MW of less than 3.9 kDa are called chitooligosaccharides (COSs), chitosan oligomers, or chitooligomers [
11,
12]. Their characteristics, such as low molecular weight, low polymerization degree, and high water solubility, are superior to those of chitin and chitosan [
11]. COS has a variety of biological activities and many potential uses in multiple fields, such as medicine, cosmetics, food, and agriculture [
13]. In addition, chemical methods using acid, hydrogen peroxide (H
2O
2)
, or sodium nitrite (NaNO
2) are also used to extract COS. There are multiple ways to obtain chitosan oligomers. These methods are categorized as enzymatic, physical, or chemical depolymerizations [
14]. Chemical methods using acid [
15,
16], H2O2 [
17], or NaNO2 [
18] physical methods, such as hydrothermal [
19], microwave [
20], ultra-sonication [
21], and gamma rays [
22]. Among the chemical methods for the hydrolysis of chitosan, acid hydrolysis is probably the best known. The enzymatic depolymerization of chitosan is characterized by the enzymes’ selective cleavage of chitosan glycosidic bonds [
23].
While hetero-chitooligosaccharides combine oligomers with varying DP, deacetylation degree (DD), and acetylation patterns (position of the N-acetyl residues in the chain), homo-chitooligosaccharides are oligomers made exclusively of either glucosamine (GlcN) or N-Acetylglucosamine (GlcNAc) units [
3,
24]. The attractive bioactive properties of COS make them suitable for various biological applications. A low molecular weight (1.5 kDa) results in aqueous solubility across a broad pH range and easy absorption through epithelial cells [
10]. The physical and biological activities of chitosan and its oligomers are governed primarily by the DP, MW, and DD [
3,
25,
26]. The most significant property of COS is its water solubility or solubility in physiological pH due to the freely accessible amino groups on the shorter chains [
27]. All heterogeneous COS with a DP < 10 and DD between 50 and 100 % are completely soluble over the pH range extending from neutral to slightly alkaline, whereas commercially available chitosan with a higher DP precipitates out at such pH values [
28].
COS exhibits superior solubility over a more extended pH range and at relatively higher concentrations than the corresponding chitosan, exhibiting exciting biological properties. Depending on their size, they are also soluble in other solvents, such as dimethyl sulfoxide, dimethylformamide, water, and alcohol. There has been a growing interest in modifying these oligomers to expand their applications. A low molecular weight (1.5 kDa) confers water solubility across a broad pH range and the capacity to be swiftly absorbed by epithelial cells. A wide variety of cells, including neuronal, embryonic, and bone-marrow-derived stem cells, can grow in COS with a degree of deacetylation (DD) > 85%, which is more compatible with cell growth than chitosan with lower DD levels [
29]. The fabrication of compatible membrane materials, in which hydrogels are the most thoroughly investigated, is a key biomedical use of COS. Hydrogels are frequently used for therapeutic purposes, including tissue engineering and drug delivery, due to their propensity to expand in aqueous and biological fluids [
30].
2. Therapeutic Applications of Chitooligosaccharides
COS possesses many biological activities and promising applications in multiple fields, such as medicine, cosmetics, food, and agriculture (Figure 4). COS, which is recognized as low MW and water soluble, is in much greater demand than its precursor molecule chitosan.
Figure 4. Therapeutic applications of chitooligosaccharides (COSs).
2.1. Chitooligosaccharides as Antioxidant Agents
Antioxidants can scavenge free radicals and protect the human body from highly toxic reactive oxygen species (ROS), slowing the progression of many chronic diseases. The ability of chitosan and its derivatives to scavenge free radicals and prevent oxidative damage by interrupting radial chain reactions is well established. Compared to chitosan, COS and its derivatives have better antioxidant properties [
58,
59]. COS antioxidant or radical-scavenging properties are primarily determined by their molecular weights and DD [
46,
58,
60]. Several chronic diseases, such as cardiovascular diseases, atherogenesis, cancer, and Parkinson’s disease, are all linked to oxidative stress [
61]. The excessive generation of ROS damages proteins, lipids, and DNA, leading to inflammation, tissue degeneration, and cellular apoptosis. ROS plays a vital role in the wound healing process at low concentrations, and an excessive level of reactive oxygen species can hinder wound healing by stimulating processes, such as inflammation and fibrosis [
62].
Though the molecular mechanism underlying COS scavenging activity is unknown, the presence of hydroxyl and free amino groups in COS is thought to be responsible for its antioxidant activity. Antioxidants are noted for their positive benefits on health by protecting cells from the harmful effects of oxidation. It was demonstrated that COS and its derivatives have a high overall reducing power and can effectively remove hydroxyl radicals and superoxide anions [
63]. The COS scavenging mechanism is believed to be based on the reaction of hydroxyl and superoxide anion radicals with active hydrogen atoms in COS, producing stable macromolecule radicals [
59,
64]. As COS can provide positrons to free radicals and transform them into more stable products, it can interrupt the chain reaction caused by free radicals [
65].
2.2. Chitooligosaccharides as Antimicrobial Agents
COS is an effective antibacterial agent that inhibits the growth of various microbes, such as bacteria, fungi, and viruses [
68,
69]. The antimicrobial activity of COS depends on the molecular weight (MW), degree of polymerization (DP), and pattern of acetylation (PA), as well as the type of organism. Though the actual mechanism of the antimicrobial activity is not clearly understood, low-molecular-weight chitosan can penetrate bacterial cell walls, bind with DNA, and inhibit DNA transcription and mRNA synthesis [
70]. Conversely, chitosan with a high molecular weight can bind to the negatively charged elements of the bacterial cell wall. As a result, it modifies the permeability of the cell, creates an impermeable layer around it, and prevents transport into the cell [
71,
72].
The acetylated sequences in the COS structure are necessary for antibacterial action, and COS with a greater number of acetylated sequences and fewer free amino groups possesses improved antimicrobial activity [
73]. It was observed that Gram-positive and Gram-negative bacteria have varied responses to the antibacterial action of COS. Gram-negative bacteria have a negatively charged cell surface; hence, the positively charged amine group in COS can severely impede their growth. However, COS does not prevent the growth of Gram-positive bacteria very effectively. The interaction between positively charged amino groups of COS and negatively charged carboxylic acid groups of bacterial cell surfaces paves the way for the formation of polyelectrolyte complexes, resulting in the formation of an impermeable coating around the bacterial cell and the suppression of metabolic activity [
74]. As a result of COS antibacterial properties, a lower pH value and more polymerization have also been deemed advantageous [
75]. Chitooligosaccharides significantly block
A. actinomycetemcomitan growth due to its effect on cell membrane permeability. The release of cellular components and the unregulated entry of substances from the surrounding environment results in microbial cell death [
69].
COS antimicrobial activities are influenced by a variety of factors, including deoxycholic acid (DA) or dicetyl phosphate (DP), as well as other physicochemical properties and microorganism types [
76]. COS can change the permeability characteristics of microbial cell membranes, preventing materials from entering or triggering the cell component’s leakage, ultimately leading to bacterial destruction. The bacterial envelope serves as the active site for COS, and membrane rupture may lead to the destruction of the microorganism. Chitosan penetration into bacterial DNA suppresses RNA transcription, an additional mechanism for killing microorganisms [
77].
COS has antibacterial properties that promote tissue granulation and collagenase activity, two key factors in wound healing. Therefore, the antibacterial property of COS is responsible for its potential to promote wound healing. The antibacterial effects of COS are superior against both Gram-positive and Gram-negative microorganisms [
78]. Antimicrobial action is enhanced with higher degrees of deacetylation than lower acetylation levels [
79]. In a study conducted in relation to yeast, chitooligosaccharides with a degree of polymerization of 32 showed strong inhibitory efficacy [
80].
2.3. Chitooligosaccharides as Anti-Inflammatory Agents
Inflammation plays a crucial role in the pathology of various diseases, such as chronic asthma, rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease, psoriasis, and cancer [
86,
87,
88,
89]. It has been demonstrated that the oral administration of COS inhibits the activation of myeloperoxidase, cyclooxygenase (COX)-2, and inducible nitric oxide synthase (iNOS), as well as the levels of proinflammatory cytokines, such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α [
90]. COS exposure may inhibit the generation of numerous proinflammatory cytokines associated with lipopolysaccharide (LPS)-induced inflammation without impairing cell viability [
91]. NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) nucleus translocation was decreased as a result of the COS suppression of LPS-induced inflammatory gene expression, which attenuated an LPS-induced inflammatory response in vascular endothelial cells [
92]. Ma et al. [
93] hypothesized that COS could reduce the phosphorylation levels of mitogen-activated protein kinases (MAPKs) and activate NF-κB and activator protein 1 (AP-1) to decrease the LPS-induced interleukin 6 (IL-6) and TNF-α generation in macrophages. In a study on the effects of COS on synoviocytes in rabbit knee joints, it was found that activating AMPK reduced the expression of the enzymes iNOS and COX-2, thereby reducing synovial inflammation [
94].
COS supplementation in the diets of animals has been shown to have a potent immunoenhancing effect. The results obtained from an evaluation of the COS effect on cyclophosphamide-induced immunosuppression by Mei et al. [
95] showed that in mice treated orally with COS and cyclophosphamide, the delayed-type hypersensitivity reaction, macrophage phagocytosis activities, and levels of cytokines IL-2, IL-12, and interferon were significantly increased, while the production of IL-10 was decreased. COS exhibited protective benefits against ovalbumin-induced lung inflammation in induced asthmatic mouse models at a maximal dose of 16 mg/kg per day, with a significant decrease in mRNA expression and protein levels of IL-4, IL-5, IL-13, and TNF-α in lung tissue and bronchoalveolar lavage fluid [
96]. The serum levels of IL-1, IL-2, IL-6, immunoglobulin (Ig) A, IgG, and IgM were all raised in early weaned pigs when COS was reintroduced into their diets. Increased cell-mediated immunity in response to weaning stress may be possible if COS can modulate the levels of specific cytokines and antibodies [
97]. COS inhibits the release of nitric oxide in LPS-induced RAW 264.7 cells and BV-2 microglia, diminishes the glycerol-induced inflammatory response in rat kidneys, and decreases organ failure in LPS-induced sepsis [
98,
99].
2.4. Chitooligosaccharides and Their Anti-Obesity Activity
Obesity is a chronic trophic metabolic disorder primarily caused by an energy imbalance, resulting in the buildup of excess body fat. Obesity is associated with type 2 diabetes (T2D), hyperlipidemia, hypertension, cerebrovascular events, and cancer [
102]. COS has excellent water solubility and lower viscosity than chitosan, and the intestine more easily absorbs it. In animal models, COS has also been shown to induce weight loss, lower triglyceride and cholesterol levels in serum, and prevent lipid buildup in hepatocytes and adipose tissues [
103,
104]. COS has demonstrated enhanced intestinal absorption, and studies have been performed to investigate its potential to reduce weight gain, blood triglyceride/cholesterol levels, and lipid buildup in the liver and adipose tissues [
25,
105,
106]. COS anti-obesity activity has been the subject of numerous hypotheses, but its precise mechanism has not been fully elucidated.
When obese rats were fed COS, higher amounts of high-density lipoproteins or cardioprotective lipid-containing particles were discovered than in control rats. These lipoproteins and particles are responsible for removing excess cholesterol from tissues and transporting them to the liver [
103]. Low-MW COS appears to be more effective in increasing plasma and hepatic lipoprotein lipase activity [
108]. The evidence obtained using mouse models suggested that COS inhibited the expression of apolipoprotein B, which reduced the amount of cholesterol found in the serum [
109]. Comparing the anti-obesity activity of COS and resistant starch (RS) to their combination, COS–RS, in rat models of induced obesity and dyslipidemia, COS–RS displayed the most significant fat and lipid-lowering benefits, followed by COS and RS [
110]. Their examination of RNA sequencing revealed an increased conversion of cholesterol to bile acids. COS reduces triglycerides, total cholesterol, low-density lipoprotein cholesterol serum levels, and the expression of endoplasmic reticulum stress pathway-related factors (GRP78, GRP94, ATF4, and CHOP), and increases oxidative lipid catabolism [
111]. This effect may be mediated by metabolites or directly by the molecules themselves. These findings underline the significance of developing COS as a potential drug derived from natural products for the prevention and treatment of obesity.
2.5. Chitooligosaccharides as Antidiabetic Agents
COS has demonstrated the potential to protect β cells from excessive glucose by promoting pancreatic cell proliferation, resulting in enhanced insulin production to lower glucose levels [
112,
113]. Reducing blood glucose levels and restoring normal insulin sensitivity were among the documented benefits of COS therapy for diabetic rats [
113]. COS possesses antidiabetic properties and may reduce diabetes mellitus (DM) incidence by affecting the glucose–lipid metabolic balance and glycemic control [
114]. Additionally, COS therapy can potentially improve the general health of diabetic rats, alleviate diabetic symptoms, bring blood glucose levels back to normal, and restore normal insulin sensitivity.
In addition, chitooligosaccharides can stimulate the multiplication of beta cells and restore the functional capacity of injured beta cells. COS has been shown to promote the overgrowth of beta cells and isolated pancreatic islet cells, in addition to increasing insulin release from pancreatic cells [
113]. Chitooligosaccharides have the potential to significantly increase the rate of proliferation of pancreatic islet cells. In diabetic mice, it was also found that dietary COS reduced hyperglycemia by activating hepatic glucokinase and increasing peripheral tissue glucose uptake, as well as by increasing pancreatic insulin secretion and improving skeletal muscle glucose uptake [
115]. In a study using streptozotocin (STZ)-induced diabetic rats, COS was able to treat hyperglycemia at a dose of 1000 mg/kg by lowering fasting serum glucose and insulin levels, thus improving O-glycosyltransferase (OGT), enhancing the index of insulin sensitivity, and reducing insulin resistance [
116]. Additionally, COS significantly increases the amount of glycogen in the liver by increasing glucokinase, which facilitates the transfer of blood glucose into liver glycogen. Low-molecular-weight COS also increases the plasma adiponectin levels in prediabetic subjects [
117].
2.6. Chitooligosaccharides in Osteoporosis
Supplemental chitooligosaccharides are beneficial in calcium-deficient states, such as osteoporosis. Supplementing animals’ diets with COS has been shown to improve calcium bioavailability in rat osteoporosis models induced by ovariectomy and concomitant low calcium intake [
121]. A substantial reduction in the serum levels of inflammatory cytokines was observed after the oral administration of COS to older persons [
122]. The mechanism behind the possible antiosteoporotic effect of COS was thought to be connected to its anti-inflammatory properties. Additionally, others have reported that COS suppressed the synthesis and expression of proinflammatory mediators in vitro [
121,
123].
The antiosteoporotic effect of COS may be partially explained by its anti-inflammatory effect via the downregulation of COX-2 expression levels, which provides additional evidence for the efficacy of selective COX-2 inhibition in preventing bone loss in estrogen-deficient animals and postmenopausal women [
124]. The mineralization process and bone density depend on Ca
2+, which provides structural support. There is evidence that COS causes an increase in bone calcium deposition [
121,
125].
2.7. The Antihypertensive Effect of COS
COS can effectively control hypertension by inhibiting renin or angiotensin-converting enzyme activity. COS is an angiotensin-converting enzyme (ACE) inhibitor since the active binding site of ACE is positively charged and contains hydrogen-bond acceptors and zinc as a cofactor. Hong et al. [
126] evaluated the ACE inhibitory actions of COS with varying degrees of polymerization from 1 to 10 and found that the chitotriose (DP = 3) derivative was the most potent. Studies have shown that the DD is inversely proportional to the ACE inhibitory activity of COS or its derivatives [
127,
128]. Carboxylated and sulfated COS have been synthesized, and it has been observed that they possess a significantly greater inhibitory action against ACE than unmodified COS [
127,
128]. It is believed that an increase in negative charges on the molecule is responsible for the improved binding of this modified COS to the integral active site of the enzyme, thereby boosting its ACE inhibitory action. The aminoethyl-conjugated COS exhibited enhanced ACE inhibitory action due to the formation of hydrogen bonds, which promotes COS binding [
129].
2.8. Chitooligosaccharides and Alzheimer’s Disease
The pathological changes in Alzheimer’s disease (AD) are caused by neuronal apoptosis, and neuronal protection is crucial in treating it [
130]. A study conducted to explore the neuroprotective effect of COS against AD showed that COS significantly decreased amyloid beta-induced cell apoptosis by reducing the expression of caspase 3 and Bax/Bcl-2 ratio activation [
131]. COS significantly reduced the neuronal damage caused by oxidative stress and glucose deprivation, and these findings imply that COS has the potential to serve as a neuroprotective agent against neurodegenerative diseases, such as Alzheimer’s disease [
132]. The protein expression and acetylcholinesterase activity produced by amyloid β peptide in PC12 cells were inhibited by COS, which was revealed to be the first stage in the pathogenic cascade of AD [
133].
2.9. Chitooligosaccharides as Antitumor Agents
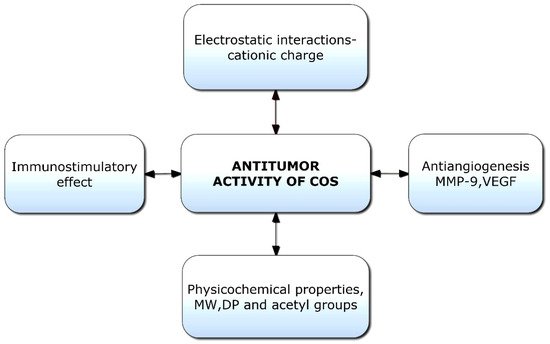
Chitooligosaccharides and their derivatives have demonstrated potent antitumor activity against human cancer cells [
136]. Though limited information is available on the antitumor mechanisms of COS, some hypotheses have been proposed (
Figure 5). Initially, the mechanism of anticancer activity was linked to the cationic character of COS; however, it was later hypothesized that relative molecular weight was also a crucial factor [
137]. The tumor-inhibiting effect of COS is likely a result of its ability to induce lymphocyte cytokines via promoting T-cell proliferation. The antitumor mechanism of COS is essentially enhanced by acquired immunity by increasing T-cell differentiation to boost cytotoxicity and preserve T-cell activity [
138]. COSs are naturally occurring polysaccharides with a cationic charge; they can promote the apoptosis of numerous cancer cells, including liver cancer, breast cancer, cervical cancer, kidney cancer, lung cancer, leukemia, and colorectal cancer cells [
25].
Figure 5. Potential factors responsible for antitumor activities of chitooligosaccharides (COSs). VEGF: Vascular endothelial growth factor; MW: Molecular weight; DP: Degree of polymerization.
2.10. Chitooligosaccharides in Wound Healing
The overexpression of miR-27a and the activation of the transforming growth factor-beta (TGF-β)-1-Smad2/3 pathway are two potential mechanisms of COS in the acceleration of wound healing and tissue regeneration [
150]. COS is combined with other biopolymers for wound healing due to its low molecular weight. In addition to its biological properties, COS can promote wound healing by enhancing a wound dressing’s water absorption, flexibility, and mechanical strength.
2.11. Chitooligosaccharides in Tissue Engineering
Cells, composite scaffolds, and signaling molecules are the three essential components for producing substituted tissues that repair, replace, or regenerate damaged tissues or organs. COS is comparable to glycosaminoglycans, a crucial part of the extracellular matrix of a cell (ECM). As it can create an environment that closely resembles the ECM, allowing for cell attachment and the preservation of growth factors, this makes it successful for scaffolds in tissue engineering applications [
156]. In the creation of bone tissue engineering scaffolds, natural polymers, such as gelatin and COS, have been used. By regulating the genes that control osteoblast proliferation in bone tissues, COS has been shown to stimulate neuronal differentiation in PC-12 nerve cells [
157]. Gelatin–COS scaffolds that were cultivated with mesenchymal stem cells (MSCs) obtained from bone marrow and capable of osteogenic differentiation demonstrated promising outcomes in the production of bone tissue [
158]. Within two weeks of implantation, cell proliferation was observed inside the scaffolds; homogenous collagen distribution within the pores and calcium deposition on the scaffolds’ surfaces indicated that the cells had successfully proliferated [
159].
2.12. Chitooligosaccharides in Drug Delivery
COS has excellent potential for usage in drug delivery systems (DDSs) due to its non-toxicity, biodegradability, and solubility in water [
154]. Due to its solubility at physiological pH, the water solubility of COS is generally more effective for drug administration [
162]. Recently, a novel alternative to red blood cells has been developed using a pectin-based COS–hydrogel microcapsule carrier designed to transport hemoglobin [
163]. Oligosaccharides were used to extend the shelf life of these therapeutic drugs by several months. With COS of a 95% degree of deacetylation (DD) and an MW of 10 kDa, non-biodegradable polyethylene glycol (PEG) and cyclodextrin inclusion complexes are converted into hydrogels capable of transporting drugs.
MW and DD are influential parameters for modulating the actions of COS, offering a strategy to improve its efficacy in drug delivery systems (DDSs) [
164]. COS is more significantly absorbed through negatively charged mucous membranes of tissues because it is more capable of binding under these situations [
165]. DD also controls binding; at a low MW, the effect of DD on charge density is more substantial [
28,
166]. This may have a positive influence on the interaction of COS with oppositely charged copolymers or active medicines; nevertheless, the considerably stronger interactions can harm cell viability [
167]. Therefore, when utilizing COS with a high DD in drug delivery, it is necessary to monitor and optimize the dosage rate to limit cytotoxic effects [
164].
This entry is adapted from the peer-reviewed paper 10.3390/polym14173558