Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
Gastric cancer is an enigmatic malignancy that has recently been shown to be increasing in incidence globally. There has been recent progress in emerging technologies for the diagnosis and treatment of the disease. Improvements in non-invasive diagnostic techniques with serological tests and biomarkers have led to decreased use of invasive procedures such as endoscopy.
- gastric cancer
- diagnosis
- management
- biomarkers
- immunotherapy
1. Introduction
As the fifth most common malignant neoplasm and the fourth most common cause of cancer-related death worldwide [1], there is no doubt that gastric cancer (GC) is a disease requiring further insight into how to better diagnose and manage it. Geographically, the incidence of gastric cancer is highest in East Asian and Eastern European regions, with around 60% of all GCs worldwide being seen in East Asia, of which 43.9% are accounted for by China alone [2]. One possible hypothesis for this pattern is that these regions are known to have a high prevalence of the established risk factors for GC, such as a different cytotoxic-associated gene A (cagA) strain of H. pylori and increased intake of salt-preserved or smoked foods [3]. Other risk factors for GC include smoking and heavy alcohol consumption [2].
2. Diagnosis
In the United States, about one-third of patients diagnosed with gastric cancer have a distant metastasis at the time of diagnosis [4]. Upper GI series and endoscopy are the gold standard and mainstay in current clinical practice for early gastric cancer diagnosis. In fact, the use of endoscopy for screening is associated with lower gastric cancer-related mortality [5]. However, in the Western world, compared to Korea and Japan where there is a high prevalence of gastric cancer, the use of upper GI endoscopy for screening is cost-ineffective and invasive, and hence other non-invasive, cost-conscious diagnostic methods are being sought [6]. Liquid biopsies have emerged as a non-invasive way of using bodily fluids (i.e., blood, peritoneal lavage, gastric juice/lavage, etc.) to provide early tumor diagnosis, assess prognosis, identify druggable targets, and monitor tumor burden while undergoing treatment [7,8]. There are several advantages to using liquid biopsies as screening for GC, one of the most obvious being its relative non-invasiveness compared with the current gold standard of endoscopy. These blood tests can detect biomarkers which include a variety of molecules that are associated with carcinogenesis of gastric cancer, including proteins, DNA, various types of RNA, exosomes, etc. Figure 1 provides a schematic for some of the liquid biopsy markers currently being studied to diagnose gastric cancer.
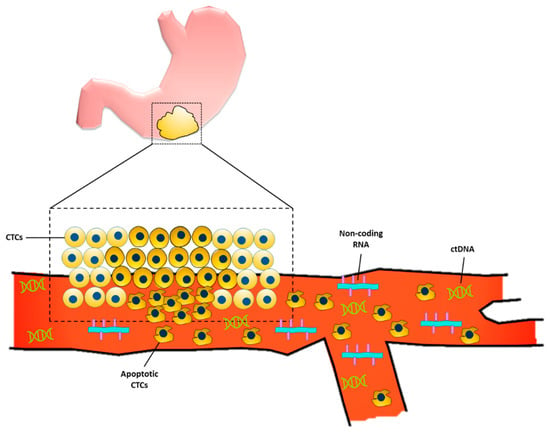
Figure 1. Liquid biopsy markers for gastric cancer. Primary gastric tumor sheds circulating tumor cells (CTCs) into the bloodstream. Some of the CTCs undergo apoptosis which allows for the release of the cell’s genetic material, including circulating tumor DNA (ctDNA) and non-coding RNAs.
2.1. Biomarkers
2.1.1. Proteins
Currently, the most widely used biomarkers for the diagnosis and monitoring of gastric cancer are carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9. Both CEA and CA19-9 have been shown to correlate with tumor burden and depth of tumor invasion [9]. However, both these markers have been proven to have low sensitivity and specificity by many studies and hence have poor diagnostic, prognostic, and monitoring values [9].
Pretreatment CA-125 is another classical prognostic marker for gastric cancer that has been associated with gastric cancer recurrence, rendering it a diagnostic tool to predict poor prognosis. Furthermore, many studies have demonstrated that elevated levels of serum CA-125 correlate with peritoneal dissemination of the primary gastric tumor. Although the sensitivity of this biomarker is poor, ranging from 19.78% [10] in some studies up to 34.3% [11], it has still been shown to have clinical utility, especially in patients with unresectable advanced or recurrent gastric cancer [11].
Another tumor marker that has been gaining significant traction for the purpose of gastric cancer screening and monitoring is CA72-4 [12]. Although there are limited studies assessing its utility, it has been shown in one study to have a higher specificity in indicating the recurrence of gastric cancer compared to CEA and CA19-19 (97% vs. 79% and 74%, respectively) (p < 0.05) [12]. However, despite its high specificity, it has been shown to have poor sensitivity and hence has limited clinical value.
Pepsinogen is another protein biomarker that can be used for the screening of gastric cancer. Pepsinogen, a precursor of pepsin, is secreted by the chief cells of the stomach and can be present in two forms, pepsinogen I (PG I), which is secreted from the fundus, and pepsinogen II (PGII), which is secreted from the pylorus and Brunner’s glands of the duodenum [13,14]. In normal conditions, the ratio between PG I and PG II is similar. In atrophic gastritis, which is a risk factor for gastric cancer since it can cause intestinal metaplasia, the destruction of fundic chief cells causes a remarkable reduction in PG I levels and hence causes a low PG I:PG II ratio [15,16]. Hence, a low PG I:PG II ratio is often used to screen for patients with atrophic gastritis which can possibly detect early gastric tumors. However, a major limitation of this biomarker is its poor sensitivity and specificity for detecting gastric tumors, which can be up to 77% and 73%, respectively, in the general population [17]. Furthermore, due to the vast variation in baseline pepsinogen levels based on ethnicity and gender, this practice has yet to be adopted into routine clinical practice [18].
Another promising biomarker is trefoil factor 3 (TFF3). This minute peptide is secreted from the goblet cells of the small and large intestine and can also be secreted from the gastric mucosa that has undergone intestinal metaplasia. Studies have shown high serum TFF3 levels to have a sensitivity of 80.9% and specificity of 81% for gastric cancer [19]. A meta-analysis of 17 studies evaluating the diagnostic value of TTF3 for GC showed that tissue TTF3 expression was associated with a higher risk of lymph node metastasis (OR 2.20, p < 0.001), muscularis propria invasion (OR 1.51, p = 0.006), and worse TNM stage (OR 2.26, p < 0.001) [20]. Furthermore, it has been shown that when combining the measurement of the PG I/PGII ratio with TFF3, there was a higher positive predictive value for detecting GC than when testing for each of the components separately [19,21]. Despite these promising results, currently there are no studies evaluating the utility of TFF3 for gastric cancer screening and diagnosis in clinical practice.
Alpha-fetoprotein (AFP) is another biomarker for gastric cancer that is most commonly seen in a rare subset of AFP-producing gastric carcinomas [22]. AFP is a glycoprotein synthesized from the embryonic yolk sac and liver during pregnancy; in clinical practice, it is most commonly used as a tumor marker for hepatocellular carcinoma [23]. Studies have found that AFP-positive GCs are characterized by a more aggressive behavior than AFP-negative GC tumors, with a higher chance of liver metastasis and venous invasion [23,24]. Hence, it is recommended that physicians routinely check AFP levels in gastric cancer patients, especially if there is concern about liver metastasis [25].
2.1.2. Circulating Tumor Cells
In 1869, a liquid biopsy was performed from the peripheral blood and provided the first known evidence for the presence of circulating tumor cells (CTCs) [26]. Liquid biopsies are samples of blood or other biological fluids that are used to detect and analyze cancer cells or cancer cell-derived molecules [27]. CTCs are cancer cells that have been released either from the primary tumor site or from the metastatic sites [28]. Several meta-analyses have demonstrated an association between the presence of GC CTCs and advanced tumor stage, lymphatic invasion, and poorer survival [29]. There are various markers for gastric cancer CTCs; for the epithelial subtype, they include EpCAm, cytokeratin (CK): CK8, CK18, and CK19 and for the mesenchymal subtype, they include vimentin and twist [30,31]. Another interesting marker for gastric cancer CTCs is fluorescence in situ hybridization (FISH) detection of CTCs with chromosome 8 aneuploidy, a mutation commonly found in gastric tumor cells [32].
Studies have shown that the clinical utility of CTCs is mainly limited to monitoring gastric tumor treatment response and prognosis rather than the early diagnosis of the tumor [28]. For example, the PRODIGE 17 trial conducted in patients with advanced gastric and esophageal cancer demonstrated that the dynamic changes in CTC count between baseline and 4 weeks after treatment were significantly associated with progression-free survival (PFS) and overall survival (OS) [33,34]. However, since CTCs are generally quickly eliminated from the body through the immune system, only a few CTCs survive in the blood circulation (around 1 CTC/mL of blood) and hence this sparsity of CTCs leads to challenges in accurately detecting their presence from liquid biopsies [35]. Due to this phenomenon as well as the heterogeneous nature of CTCs, various CTC detection methods have yielded different detection rates.
2.1.3. Circulating Tumor DNA
Circulating tumor DNA (ctDNA) is another biomarker that can be extracted from liquid biopsies for the diagnosis and monitoring of gastric cancer. CtDNA can be produced from primary tumor cells, CTCs, or a distant metastasis and it can give a wide range of information on the malignancy, such as methylation status and other genetic alterations [28]. Fang et al. demonstrated a correlation in gastric cancer patients between ctDNA levels and vascular invasion, 5-year survival rate, and peritoneal recurrence [36]. Furthermore, a meta-analysis of 16 studies showed a significant association between the presence of ctDNA with worse OS (p < 0.001) and DFS (p < 0.001) [37]. The study also showed that ctDNA levels had a significant association with TNM stage, tumor depth, lymph node metastasis, and distant metastasis with a specificity of 95% and a sensitivity of 62%. However, due to the complex technology required to detect ctDNA within the plasma, it has yet to be of significant use in clinical practice [28].
2.1.4. Non-Coding RNA
Non-coding RNAs (ncRNAs) are types of RNA that do not encode protein and can be classified into two subgroups: small ncRNAs (sncRNAs) and long ncRNAs (lncRNAs). The sncRNAs can be further subclassified into microRNAs (miRNAs), small nuclear RNAs (snRNAs), and piwi-interacting RNAs (piRNAs). These various non-coding RNAs can also be used in the detection and monitoring of gastric cancer.
miRNAs play a crucial role in various cellular functions through regulating epigenetic mechanisms; these functions include cellular growth, apoptosis, differentiation, and even gastric tumor carcinogenesis. Wu et al. studied 50 GC patients and 50 patients without GC and found an increase in levels of miRNA-21 in patients with GC compared to those who did not have it (p < 0.01), with a sensitivity of 81.3% and a specificity of 73.4% [38]. Hung et al. demonstrated increased levels of miRNA-376c in the tissue, plasma, and urine of GC patients owing to the fact that miRNA-37c was found to increase the proliferation, migration, and anchorage-independent growth of carcinoma cells [39]. Other panels of miRNA that have also been shown to be upregulated include miRNA-196a [40], -200c [41], -375 [42], -940 [43], and many others [28]. Despite these promising results, the clinical utility of miRNA in routine practice has many current limitations. For example, there can be inaccuracies in miRNA quantification due to variations in processing, storage, RNA extraction, and reference gene choice during qRT-PCR since there is no unique protocol developed yet to control these parameters [32]. More details about the utility of miRNA in the diagnosis of GC are outlined in the next section on circulating extracellular vesicles.
Another sncRNA that is used as a biomarker in the diagnosis of gastric cancer is piRNAs. This newly discovered type of non-coding RNA has been shown to be a molecule that is not easily degraded and able to be detected in various human bodily fluids, including serum and gastric juice [44]. Cui et al. [45] performed a peripheral blood test in both healthy and GC patients and showed that GC patients had lower levels of piRNA-651 and piRNA-823 compared to their healthy counterparts. These results also had relatively high sensitivity and specificity of 94.9% and 96.4%, respectively. Due to these promising results, further studies and clinical trials are being conducted to better understand the clinical utility of piRNAs as potential biomarkers for gastric cancer [46].
LncRNAs have also been proposed as biomarkers for GC. For example, a lncRNA called “high up-regulated in liver cancer” (HULC) has been shown to be increased in the serum of GC patients compared to normal controls [47]. Likewise, the lncRNA H19 also showed similar results [48]. Supporting this evidence, both serum HULC and H19 were shown to be significantly decreased in post-treatment GC patients compared to levels obtained prior to treatment. The sensitivity for HULC and H19 was 82% and 74%, respectively, while the specificity for both molecules was 83.6% and 58%, respectively. The clinical application limitations are similar to those of miRNA.
2.1.5. Circulating Extracellular Vesicles
Extracellular vesicles (EVs), also known as exosomes, are small spherical structures with an outer lipid bilayer that are secreted from cells into the extracellular space, and participate in inter-cellular communication through the transfer of functional molecules scavenged and secreted into EVs [8,49]. Exosomes are a type of EV measuring 40–120 nm that are produced in the endosomal compartment of the cell [50]. The contents of these exosomes include proteins, miRNAs, lncRNAs, etc. GC-derived exosomes can communicate with cells in the tumor microenvironment, allowing it to become more favorable in establishing metastatic niches. These exosomes also suppress host innate and adaptive immune responses by regulating host immunomodulatory mediators [8,51].
Some exosomal proteins are involved in the development of GC. TGF-β1 is an immunosuppressive cytokine that has been detected in exosomes of GC patients and was found to be correlated with lymphatic metastasis [52,53]. Tripartite motif 3 (TRIM3) is a protein that normally inhibits the proliferation of GC cells; it has been found that the levels of TRIM3 in serum exosomes of patients with GC are lower than those of healthy controls, making it a potential diagnostic biomarker for GC [54]. Gastrokine-1 (GKN-1) is another exosomal cargo protein involved in regulating the immune response and inhibiting proliferation of GC cells [54]. Yoon et al. found that healthy controls had significantly higher serum GKN1 levels than GC patients (6.34 ng/μL vs. 3.48 ng/μL, p < 0.0001), suggesting it to be another potential biomarker for GC. Heat shock proteins (HSP) 60 and 70 have been found in higher concentration within exosomes derived from malignant ascites in GC patients compared with exosomes derived from ascites in non-GC patients [55]. HSP-60 and 70 aid in the immune response against GC by promoting the maturation of dendritic cells, inducing a cytotoxic T-lymphocyte response against the tumor [54]. These exosomal proteins have yet to be studied in large cohort clinical trials and hence their applicability in the clinical setting is yet to be known.
The use of exosomal DNA for the diagnosis and prognosis of GC is an area of research rarely targeted in the literature. There have been only three studies investigating this up until now with only four exosomal genes identified in relation with GC so far: BARHL2, LINE1, SOX17, and miRNA-34b/c gene. Gastric juice-derived exosomal BARHL2 gene methylation was suggested to have promising potential as a biomarker with GC patients being more likely to have BARHL2 methylation compared to non-GC controls (90% sensitivity and 100% specificity) [56]. Another study also investigating the detection of methylated DNA in gastric juice-derived exosomes found that patients with GC had reduced LINE1 methylation whereas SOX17 gene methylation was detected in both early and advanced gastric cancer of both intestinal and diffuse type [57]. These findings suggest the promising potential of gastric juice-derived exosomal DNA for the early detection of GC in the clinical setting.
It has been proposed that exosomal miRNAs have promising potential as diagnostic molecules for GC tumors. Ren et al. extracted exosomes from GC cell lines and non-GC cell lines and found that the exosomes of GC cell lines contained higher levels of miRNA-21-5p and miRNA-30-p compared to the non-GC cell lines [58]. Another study by Wang et al. found that exosomal miRNA-19b-3p and exosomal miRNA-106a-5p had 95% sensitivity and 90% specificity in detecting GC, suggesting them to be promising biomarkers for the diagnosis of GC [59]. Huang et al. identified six miRNAs that were significantly upregulated in the serum of GC patients, with four of them (miRNA-10b-5p, miRNA-195-5p, miRNA-20a-3p, and miRNA-296-5p) showing significant upregulation in serum exosomes [60]. Furthermore, Tokuhisa et al. found that miRNA-1225-5p and miRNA-21 from peritoneal lavage fluid were upregulated in the later stages of GC and correlated with serosal invasion, which could potentially predict peritoneal recurrence following curative GC resection [61]. Despite these extensive findings on the potential utility of exosomal miRNAs for the diagnosis and prognosis of GC, there have yet to be any clinical trials to investigate this further in the clinical setting and hence their applicability in the real word is yet to be determined [51,62].
This entry is adapted from the peer-reviewed paper 10.3390/cancers14225615
This entry is offline, you can click here to edit this entry!
