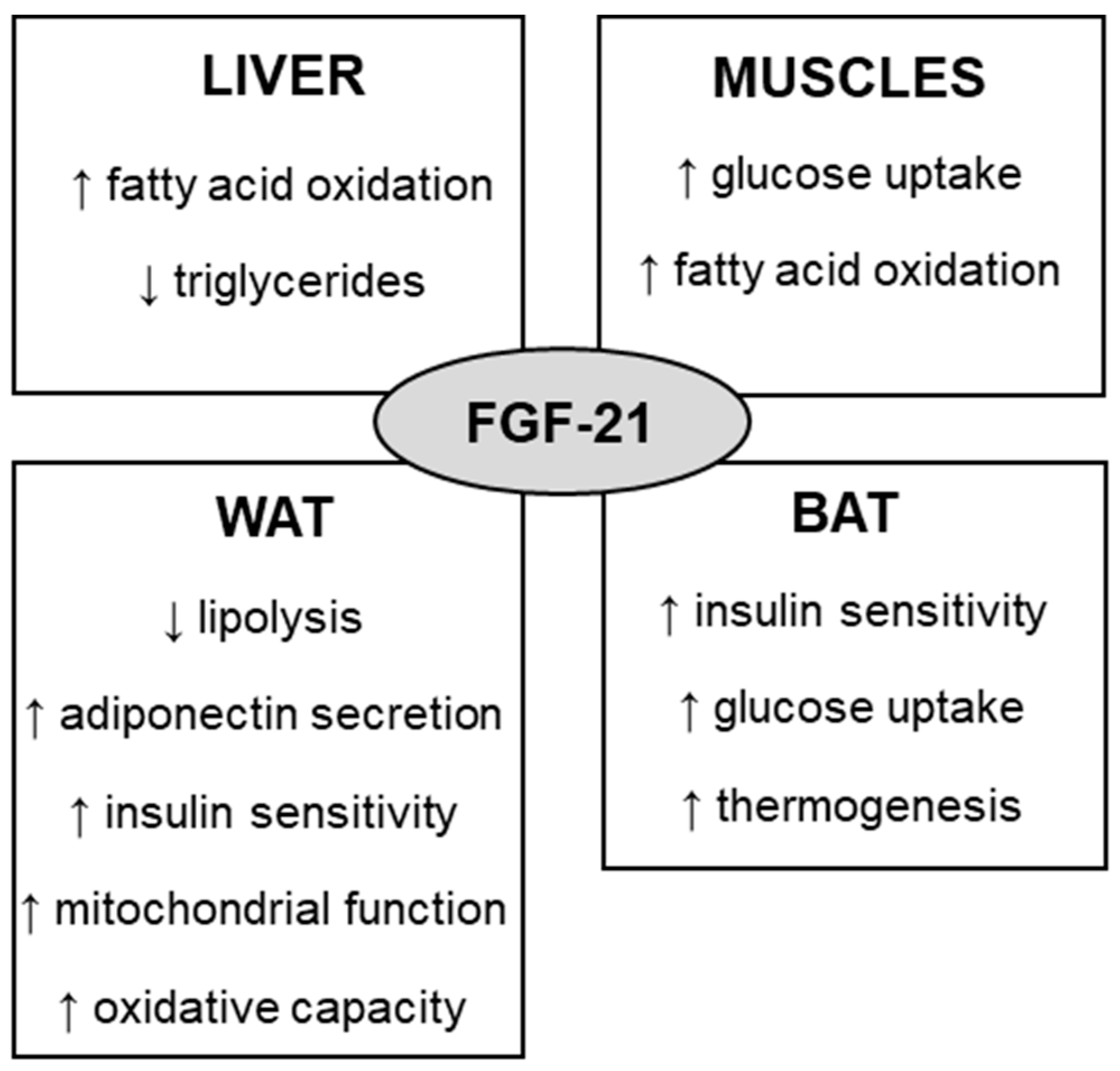
Fibroblast growth factor 21 (FGF-21) is a protein that is involved in the regulation of glucose, lipids, and energy metabolism. To act on target tissues, endocrine FGF-21 binds preferably to FGF receptor 1 (FGFR1) in the presence of the coreceptor named β-klotho (KLB). Some of the effects of FGF-21 include increased fatty acid oxidation, glucose uptake, insulin sensitivity, and thermogenesis, which can regulate body weight and glycemia control. By exerting such metabolic effects, the therapeutic potential of FGF-21 for the treatment of obesity and diabetes has been investigated. Physical exercise has been widely used for the prevention and treatment of obesity. Several mechanisms mediate the effects of physical exercise, including the FGF-21 pathway. Studies have shown that physical exercise increases the concentration of circulating and tissue FGF-21 in animals, while contradictory results are still observed in humans.
- FGF-21
- physical exercise
- obesity
1. Introduction
2. FGF-21 Metabolic Effects
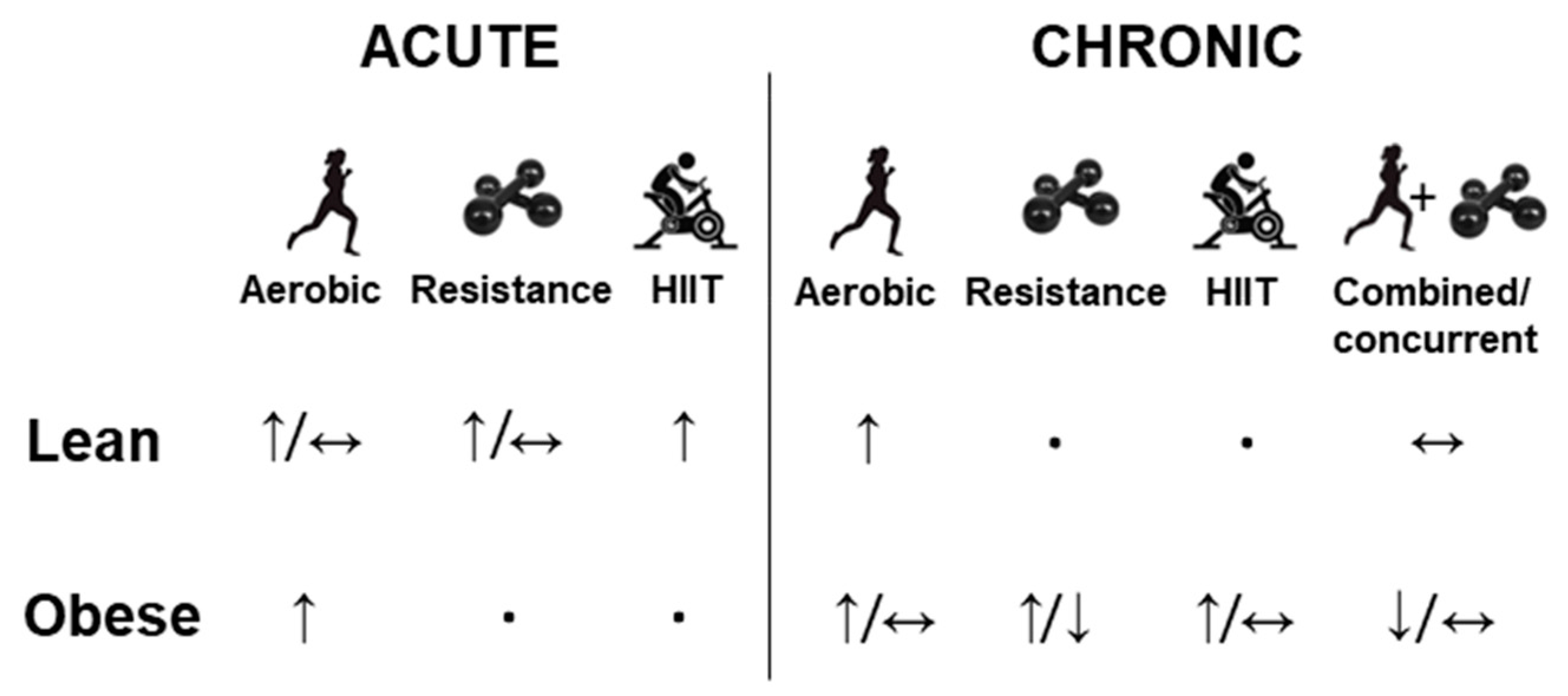
3. Effect of Physical Exercise on FGF-21
This entry is adapted from the peer-reviewed paper 10.3390/obesities2040031
References
- Staiger, H.; Keuper, M.; Berti, L.; de Angelis, M.H.; Häring, H.U. Fibroblast growth factor 21-metabolic role in mice and men. Endocr. Rev. 2017, 38, 468–488.
- Itoh, N.; Ohta, H.; Konishi, M. Endocrine FGFs: Evolution, Physiology, Pathophysiology, and Pharmacotherapy. Front. Endocrinol. 2015, 6, 154.
- Huang, Z.; Xu, A.; Cheung, B.M.Y. The Potential Role of Fibroblast Growth Factor 21 in Lipid Metabolism and Hypertension. Curr. Hypertens. Rep. 2017, 19, 17–22.
- Charoenphandhu, N.; Suntornsaratoon, P.; Krishnamra, N. Fibroblast growth factor—21 restores insulin sensitivity but induces aberrant bone microstructure in obese insulin—Resistant rats. J. Bone Miner. Metab. 2016, 35, 142–149.
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 2005, 115, 1627–1635.
- Coskun, T.; Bina, H.A.; Schneider, M.A.; Dunbar, J.D.; Hu, C.C.; Chen, Y.; Moller, D.E.; Kharitonenkov, A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 2008, 149, 6018–6027.
- Jimenez, V.; Jambrina, C.; Casana, E.; Sacristan, V.; Muñoz, S.; Darriba, S.; Rodó, J.; Mallol, C.; Garcia, M.; León, X.; et al. FGF21 gene therapy as treatment for obesity and insulin resistance. EMBO Mol. Med. 2018, 10, e8791.
- Venables, M.C.; Jeukendrup, A.E. Endurance training and obesity: Effect on substrate metabolism and insulin sensitivity. Med. Sci. Sports Exerc. 2008, 40, 495–502.
- Winding, K.M.; Munch, G.W.; Iepsen, U.W. The effect of low-volume high-intensity interval training versus endurance training on glycemic control in individuals with type 2 diabetes. Diabetes Obes. Metab. 2018, 20, 1131–1139.
- Higa, T.S.; Spinola, A.V.; Fonseca-Alaniz, M.H.; Evangelista, F.S. Remodeling of white adipose tissue metabolism by physical training prevents insulin resistance. Life Sci. 2014, 103, 41–48.
- Américo, A.L.V.; Muller, C.R.; Vecchiatto, B.; Martucci, L.F.; Fonseca-Alaniz, M.H.; Evangelista, F.S. Aerobic exercise training prevents obesity and insulin resistance independent of the renin angiotensin system modulation in the subcutaneous white adipose tissue. PLoS ONE 2019, 14, e0215896.
- Muller, C.R.; Américo, A.L.V.; Fiorino, P.; Evangelista, F.S. Aerobic exercise training prevents kidney lipid deposition in mice fed a cafeteria diet. Life Sci. 2018, 211, 140–146.
- Vecchiatto, B.; da Silva, R.C.; Higa, T.S.; Muller, C.R.; Américo, A.L.V.; Fortunato-Lima, V.C.; Ferreira, M.M.; Martucci, L.F.; Fonseca-Alaniz, M.H.; Evangelista, F.S. Oxidative phenotype induced by aerobic physical training prevents the obesity-linked insulin resistance without changes in gastrocnemius muscle ACE2-Angiotensin(1-7)-Mas axis. Diabetol. Metab. Syndr. 2021, 13, 74.
- She, Q.-Y.; Bao, J.; Wang, H.; Liang, H. Fibroblast growth factor 21: A “rheostat” for metabolic regulation? Metabolism. 2022, 130, 155166.
- Badman, M.K.; Pissios, P.; Kennedy, A.R.; Koukos, G.; Flier, J.S.; Maratos-Flier, E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007, 5, 426–437.
- Inagaki, T.; Dutchak, P.; Zhao, G.; Ding, X.; Gautron, L.; Parameswara, V.; Li, Y.; Goetz, R.; Mohammadi, M.; Esser, V.; et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007, 5, 415–425.
- Ding, X.; Boney-Montoya, J.; Owen, B.M.; Bookout, A.L.; Coate, K.C.; Mangelsdorf, D.J.; Kliewer, S.A. βKslotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell Metab. 2012, 16, 387–393.
- Geng, L.; Lam, K.S.L.; Xu, A. The therapeutic potential of FGF21 in metabolic diseases: From bench to clinic. Nat. Rev. Endocrinol. 2020, 16, 654–667.
- Zhang, X.; Yeung, D.C.Y.; Karpisek, M.; Stejskal, D.; Zhou, Z.-G.; Liu, F.; Wong, R.L.C.; Chow, W.-S.; Tso, A.W.K.; Lam, K.S.L.; et al. Serum FGF21 Levels Are Increased in Obesity and Are Independently Associated With the Metabolic Syndrome in Humans. Diabetes 2008, 57, 1246–1253.
- Hale, C.; Chen, M.M.; Stanislaus, S.; Chinookoswong, N.; Hager, T.; Wang, M.; Véniant, M.M.; Xu, J. Lack of Overt FGF21 Resistance in Two Mouse Models of Obesity and Insulin Resistance. Endocrinology 2012, 153, 69–80.
- Fisher, f.M.; Chui, P.C.; Antonellis, P.J.; Bina, H.A.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E. Obesity Is a Fibroblast Growth Factor 21 (FGF21)-Resistant State. Diabetes 2010, 59, 2781–2789.
- Spann, R.A.; Morrison, C.D.; den Hartigh, L.J. The Nuanced Metabolic Functions of Endogenous FGF21 Depend on the Nature of the Stimulus, Tissue Source, and Experimental Model. Front. Endocrinol. 2022, 12, 1–21.
- Kaufman, A.; Abuqayyas, L.; Denney, W.S.; Tillman, E.J.; Rolph, T. AKR-001, an Fc-FGF21 Analog, Showed Sustained Pharmacodynamic Effects on Insulin Sensitivity and Lipid Metabolism in Type 2 Diabetes Patients. Cell Reports Med. 2020, 1, 100057.
- Turer, A.T.; Scherer, P.E. Adiponectin: Mechanistic insights and clinical implications. Diabetologia 2012, 55, 2319–2326.
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295.
- Xu, A.; Wang, Y.; Keshaw, H.; Xu, L.Y.; Lam, K.S.L.; Cooper, G.J.S. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J. Clin. Investig. 2003, 112, 91–100.
- Babaknejad, N.; Nayeri, H.; Hemmati, R.; Bahrami, S.; Esmaillzadeh, A. An Overview of FGF19 and FGF21: The Therapeutic Role in the Treatment of the Metabolic Disorders and Obesity. Horm. Metab. Res. 2018, 50, 441–452.
- Khalafi, M.; Alamdari, K.A.; Symonds, M.E.; Nobari, H.; Carlos-Vivas, J. Impact of acute exercise on immediate and following early post-exercise FGF-21 concentration in adults: Systematic review and meta-analysis. Hormones 2021, 20, 23–33.
- He, Z.; Tian, Y.; Valenzuela, P.L.; Huang, C.; Zhao, J.; Hong, P.; He, Z.; Yin, S.; Lucia, A. Myokine Response to High-Intensity Interval vs. Resistance Exercise: An Individual Approach. Front. Physiol. 2018, 9, 1735.
- Sargeant, J.A.; Aithal, G.P.; Takamura, T.; Misu, H.; Takayama, H.; Douglas, J.A.; Turner, M.C.; Stensel, D.J.; Nimmo, M.A.; Webb, D.R.; et al. The influence of adiposity and acute exercise on circulating hepatokines in normal-weight and overweight/obese men. Appl. Physiol. Nutr. Metab. 2018, 43, 482–490.
- Cuevas-Ramos, D.; Almeda-Valdés, P.; Meza-Arana, C.E.; Brito-Córdova, G.; Gómez-Pérez, F.J.; Mehta, R.; Oseguera-Moguel, J.; Aguilar-Salinas, C.A. Exercise increases serum fibroblast growth factor 21 (FGF21) levels. PLoS ONE 2012, 7, e38022.
- Parmar, B.; Lewis, J.E.; Samms, R.J.; Ebling, F.J.P.; Cheng, C.C.; Adams, A.C.; Mallinson, J.; Cooper, S.; Taylor, T.; Ghasemi, R.; et al. Eccentric exercise increases circulating fibroblast activation protein α but not bioactive fibroblast growth factor 21 in healthy humans. Exp. Physiol. 2018, 103, 876–883.
- He, Z.; Tian, Y.; Valenzuela, P.L.; Huang, C.; Zhao, J.; Hong, P.; He, Z.; Yin, S.; Lucia, A. Myokine/adipokine response to “aerobic” exercise: Is it just a matter of exercise load? Front. Physiol. 2019, 10, 691.
- Ost, M.; Coleman, V.; Kasch, J.; Klaus, S. Regulation of myokine expression: Role of exercise and cellular stress. Free Radic. Biol. Med. 2016, 98, 78–89.
- Mendez-Gutierrez, A.; Aguilera, C.M.; Osuna-Prieto, F.J.; Martinez-Tellez, B.; Rico Prados, M.C.; Acosta, F.M.; Llamas-Elvira, J.M.; Ruiz, J.R.; Sanchez-Delgado, G. Exercise-induced changes on exerkines that might influence brown adipose tissue metabolism in young sedentary adults. Eur. J. Sport Sci. Online ahead of print. 2022.
- Domin, R.; Dadej, D.; Pytka, M.; Zybek-Kocik, A.; Ruchała, M.; Guzik, P. Effect of Various Exercise Regimens on Selected Exercise-Induced Cytokines in Healthy People. Int. J. Environ. Res. Public Health 2021, 18, 1261.
- Worku, M.G.; Seretew, W.S.; Angaw, D.A.; Tesema, G.A. Prevalence and Associated Factor of Brown Adipose Tissue: Systematic Review and Meta-Analysis. Biomed Res. Int. 2020, 2020, 9106976.
- Otero-Díaz, B.; Rodríguez-Flores, M.; Sánchez-Muñoz, V.; Monraz-Preciado, F.; Ordoñez-Ortega, S.; Becerril-Elias, V.; Baay-Guzmán, G.; Obando-Monge, R.; García-García, E.; Palacios-González, B.; et al. Exercise Induces White Adipose Tissue Browning Across the Weight Spectrum in Humans. Front. Physiol. 2018, 9, 1781.
- Norheim, F.; Langleite, T.M.; Hjorth, M.; Holen, T.; Kielland, A.; Stadheim, H.K.; Gulseth, H.L.; Birkeland, K.I.; Jensen, J.; Drevon, C.A. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014, 281, 739–749.
- Koh, Y.J.; Lee, J.H.; Park, S.Y. Moxibustion-simulating bipolar radiofrequency suppresses weight gain and induces adipose tissue browning via activation of UCP1 and FGF21 in a mouse model of diet-induced obesity. Evid.-Based Complement. Altern. Med. 2018, 2018, 4737515.
