Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Cardiovascular disease (CVD) is the number one cause of death worldwide. Evidence has demonstrated an association between the gut microbiota and CVD, including heart failure, cerebrovascular illness, hypertension, and stroke. Marine algal polysaccharides (MAPs) are valuable natural sources of diverse bioactive compounds. MAPs have many pharmaceutical activities, including antioxidant, anti-inflammatory, immunomodulatory, and antidiabetic effects.
- cardiovascular diseases
- marine algae polysaccharides
- gut microbiota
1. Introduction
Cardiovascular disease (CVD) has become a major disease that threatens the health of human beings. CVD is a leading cause of death and morbidity worldwide. In China, approximately 40% deaths are attributed to CVD, which is higher than the cancer death rate or death rate from other diseases. CVD was a main cause of death in 2016, accounting for 45.50% in rural areas and 43.16% in cities [1]. The prevalence of CVD in China has been continuously increasing, and an upward trend will continue in the future. CVD includes coronary heart disease, heart failure, cardiomyopathy, hypertension, and stroke. The prevalence of CVD is also quickly rising in other developing nations (such as India and the African and Latin American continents), primarily due to diseases associated with atherosclerosis. In addition, viral and postinfectious diseases, which are also common in underdeveloped nations, have a negative impact on the heart and blood vessels [2]. In the USA, CVD is also the leading cause of mortality. Although the death rate of people with CVD has fallen over the previous few decades, nearly 2200 individuals died in the USA of CVD each day in 2016 [3]. According to estimates, 92.1 million people in the USA have at least one type of CVD, and by 2030, it is predicted that 44% of U.S. adults will suffer from more than one type of CVD [4]. As for Europe, Australia, and some developed countries in South America, the mortality rates of CVD are the lowest in the world. Despite this, CVD remains the main cause of death in Europe, approximately 42% and 52% for men and women each year [5]. The actual risk factors for CVD are becoming clearer, including high blood pressure, diabetes, obesity, high consumption of alcohol, inadequate physical activity, and unhealthy foods [6]. Frequent exposure to unhealthy foods, such as those high in sugar, fat, and salt, and a low consumption of dietary fiber leads to the gut microbiome’s dysbiosis. However, while still not fully understood, it has been recognized that the complex interactions between dietary fibers and the intestinal flora and gut-generated metabolites may play a significant role in CVD. There is increasing research attention to the gut microbiota as a strategy for the protection and treatment of CVD.
The human gut microbiome is a complex community predominantly found in the large intestine. Bacteria in the colon comprise hundreds of species and are present at levels of approximately 1011–1012 cells per mL of colonic content [7]. The majority of bacteria come from two phyla, Bacteroidetes and Firmicutes [8]. The gut microbiota is involved in degrading otherwise indigestible food components in the upper gastrointestinal tract, such as polysaccharides, into compounds that the host can absorb, and this process yields numerous functional metabolites, several of which have beneficial effects on the intestinal barrier and cardiovascular health. In addition, a rich and diverse gut microbiome leads to a well-balanced and healthy composition. A healthy gut ecosystem is critical to immune function and preventing disease development. Gut dysbiosis may play a role in a number of diseases; first and foremost are gastrointestinal diseases, which are the most studied, followed by those related to metabolic syndromes. There is also evidence that CVD is strongly correlated with the intestinal flora. Because of the complexity of the intestinal flora, researchers remain challenged to explore the roles of the intestinal flora and their functional metabolites in potential applications for the management of human health and CVD conditions.
Marine algae are far more abundant than other resources in the oceans. Marine algae contain a wide range of nutrients, including polysaccharides, proteins, peptides, amino acids, mineral salts, lipids, and polyphenols [9]. Among various nutrients, polysaccharides are the main constituents and key biological compounds in marine algae [10]. Lately, there has been an upsurge of interest in the pharmaceutical activities and potential applications of marine algal polysaccharides (MAPs). Several researchers have demonstrated that MAPs have anti-inflammatory, antioxidant, immunomodulatory, antitumor, antibacterial, and antidiabetic activities in vivo, in vitro, and in clinical experiments [11][12][13][14][15][16]. MAPs are not digested directly in the upper gastrointestinal tract but can be utilized through fermentation by gut microorganisms [17]. This process may improve the gut microflora profile (such as the composition, diversity, and richness) and stimulate the production of functional metabolites by commensal bacteria.
2. Cardioprotective Effect of MAPs Associated with Gut Microbiota Modulation
Predictably, gut microbiota dysbiosis is most often associated with gastrointestinal disorders, which in turn influence the host immune response. Emerging evidence suggests an interaction between the gut microbiome and CVD, including atherosclerosis, hypertension, peripheral artery disease, atrial fibrillation, myocardial fibrosis, and heart failure [18]. Many studies have reported alterations in the diversity and composition of the gut bacteria in humans with CVD, as summarized in Table 1. For example, the obesity component, which is a CVD risk factor, is involved in the development of dysbiotic gut microbiota, an imbalance favoring Firmicutes and Bacteroidetes in obesity in humans and animals [19].
Bacteroides species are anaerobic gut commensals in humans and are recognized as conferring a myriad of benefits to the human host. Among them is the provision of energy from a variety of MAPs that are known to produce several secondary metabolites that are beneficial to the intestinal mucosal layer and decorate the surface of other microbes [20][21]. Bacteroides species exhibit genome-encoded carbohydrate active enzymes (CAZymes), which can hydrolyze glycoside linkages by following the release of the reducing sugars from MAPs [22]. CAZymes have five classes of carbohydrases: glycoside hydrolase (GH), glycosyltransferase (GT), polysaccharide lyase (PL), carbohydrate esterase (CE), auxiliary activities, and carbohydrate-binding module (CBM). Nguyen et al. reported a significant increase in Bacteroides with higher energy metabolism due to a laminarin-supplemented high-fat diet [23]. They showed a higher abundance of CAZymes in laminarin-supplemented high-fat diet mice compared to high-fat diet mice, with especially dramatic increases in glycoside hydrolases and polysaccharide lyases, including GT2, GT4, GH2, CE8, CE12, PL1, and PL10. Porphyran from Porphyra haitanensis decreased lipid accumulation and maintained gut microbiota homeostasis in diet-induced obese mice, including an increase in the relative proportion of Bacteroides, Roseburia, and Eubacterium and a marked reduction in Helicobacter.
Firmicutes and Bacteroidetes represent greater than 90% of the total bacteria community, while the Firmicutes/Bacteroidetes ratio was reported to be increased in spontaneously hypertensive rats [19]. The Firmicutes/Bacteroidetes ratio has emerged as a possible characteristic for gut dysbiosis and CVD risk factors. A low Firmicutes/Bacteroidetes ratio was found to be strongly correlated with a balanced immune status and is generally considered beneficial for health [24]. Several studies have demonstrated that polysaccharides derived from natural resources efficiently reduced the Firmicutes/Bacteroidetes ratio. Sargassum pallidum polysaccharides modulated the gut microbiota composition by decreasing the ratio of Firmicutes/Bacteroidetes and enhancing the relative proportion of some beneficial genera, such as Bacteroides, Dialister, Phascolarctobacterium, Prevotella, and Ruminococcus when investigated by in vitro fermentation assay [25]. MAPs treatment can reduce the ratio of Firmicutes/Bacteroidetes, as verified in an in vivo CVD risk model. The green alga Enteromorpha prolifera polysaccharide showed anti-hyperuricemic effects, including significantly lowering the level of serum uric acid, xanthine oxidase, and blood urea nitrogen. Enteromorpha prolifera polysaccharide maintained the stability of the intestinal flora and showed a significant decrease in the Firmicutes/Bacteroidetes ratio [26].
An increase in the growth of Akkermansia muciniphila has been found to be favorable for the prevention of type 2 diabetes, obesity, atherosclerosis, and other metabolic syndromes. Akkermansia is the only genus of the phylum Verrucomicrobia found in gastrointestinal samples. In high-fat diet-induced obese mice, the abundance of Akkermansia muciniphila was strongly correlated with the expression of fat metabolism and inversely related with inflammation in adipose tissue and circulating glucose, adiponectin, leptin, triglycerides, and insulin [27]. Shang et al. reported that Enteromorpha clathrata polysaccharides dramatically elevated the relative abundance of Akkermansia muciniphila, Bifidobacterium spp., and Lactobacillus spp. in the gut [28]. In addition, they demonstrated a similar beneficial pharmacological effect of two fucoidans from Laminaria japonica and Ascophyllum nodosum on diet-induced metabolic damage in the C57BL/6 J mice model, which increased the abundance of Akkermansia muciniphila, Alloprevotella, Blautia, and Bacteroides treatment by fucoidan [29].
Bifidobacterium and Lactobacillus species are well-known probiotics that beneficially affect the host organism by improving and modulating the intestinal flora and preventing CVD [30]. Bifidobacterium and Lactobacillus strains possess different degrees of cholesterol removal from the media through cholesterol assimilation during growth, the binding capacity of cholesterol to cells, the incorporation of cholesterol into the cytoplasmic membrane, and bile salt deconjugation [31]. Alginate oligosaccharide treatment improved fat metabolism and inflammation by regulating the intestinal microbiota in high-fat diet-induced gut dysbiosis mice, especially by increasing the abundance of Lactobacillus gasseri and Lactobacillus reuteri [32]. Undaria pinnatifida polysaccharide intervention significantly reduced the fasting blood glucose levels, mitigated the impaired glucose tolerance, and improved insulin resistance in diabetic rats by promoting the growth of beneficial bacteria, such as Bifidobacterium, Lactobacillus, Faecalibaculum, Lachnoclostridium, and Olsenella [33].
MAPs play an important role in the intestinal microenvironment reconstruction, based on both richness and diversity. Researchers suggest that the therapeutic manipulation of the intestinal microbiota with MAPs may be a successful treatment selection to restore gut microbiota composition and potentiate heart failure management (Figure 1). Undigested MAP can be fermented by intestinal microbiota for their growth and the production of secondary metabolites, such as SCFAs, bile acids, and TMA.
Table 1. Marine algal polysaccharides and their effect on CVD prevention through gut microbiota.
| Type of Polysaccharides | Marine Algae Sources | Influence on Intestinal Microbiota | Treatment and Prevention of CVD | Ref. |
|---|---|---|---|---|
| alginate | Sargassum fusiforme | Lactobacillus, Bacteroides, Akkermansia Alloprevotella, Weissella, and Enterorhabdus ↑ Turicibacter and Helicobacter ↓ |
attenuated pathological changes in adipose, hepatic, and heart tissues; diminished oxidative stress | [34] |
| carrageenan | Kappaphycus Alvarezii | Parasutterella, Alloprevotella, Oscillibacter, Melainabacteria, and Butyricimonas ↑ Clostridia, Erysipelotrichaceae, Blautia, and Lachnospiraceae ↓ |
decreased total cholesterol and high-density level cholesterol; reduced adipocyte size and levels of adiponectin and leptin | [35] |
| fucan | Saccharina japonica | Bacteroides sartorii, Bacteroides acidifaciens, Akkermansia, and Lachnospiraceae NK4A136 ↑ | prevented high-fat diet-induced obesity; regulated blood glucose/lipid metabolism | [36] |
| fucoidan | Laminaria japonica | phylum Bacteroidetes and families Muribaculaceae and Bacteroidaceae ↑ | ameliorated high-fat diet-induced body weight gain, fat accumulation, serum lipid profiles, insulin resistance, hepatic steatosis, and adipocyte hypertrophy | [37] |
| fucoidan | Sargassum fusiforme | Bacteroides, Faecalibacterium, and Blautia ↑ | reduced epididymal fat deposition, decreased oxidative stress, and attenuated the pathological changes in heart tissues | [38] |
| fucoidan | Sargassum fusiforme | Bacteroides, Ruminococcaceae, and Butyricoccus↑ Helicobacter↓ |
reduced fat accumulation; enhanced the energy expenditure through increasing the expression of uncoupling protein 1 in adipose tissues | [39] |
| porphyran | Porphyra haitanensis | Roseburia and Eubacterium ↑, Helicobacter ↓ | ameliorated body fat accumulation in liver, serum, and adipose tissues; increased the pathway of PGC 1α-UCP 1-mitochondrial to produce more energy |
[40] |
| porphyran | Neoporphyra haitanensis | Parabacteroides and Coriobacteriaceae UCG-002 ↑ | inhibited G6Pase and PEPCK enzymes related to hepatic gluconeogenesis; enhanced the expression of the GLUT4 enzyme involved in peripheral glucose uptake | [41] |
| ulvan | Enteromorpha prolifera | Desulfovibrio ↑, modulated Verrucomicrobiaceae, Odoribacteraceae, Mogibacteriaceae, Planococcaceae, and Coriobacteriaceae | decreased levels of inflammatory factors, including IFN-γ, TNF-α, and IL-6; increased total antioxidant capacity and superoxide dismutase, glutathione, catalase, and telomerase levels | [42] |
| ulvan | Ulva lactuca | Dubosiella, Lactobacillus, and Parasutterella ↑ Staphylococcus, Escherichia−Shigella, and Ruminococcus ↓ |
reduced the amount of blood urea nitrogen, serum uric acid, and creatinine; suppressed the activities of serum and hepatic xanthine oxidase | [43] |
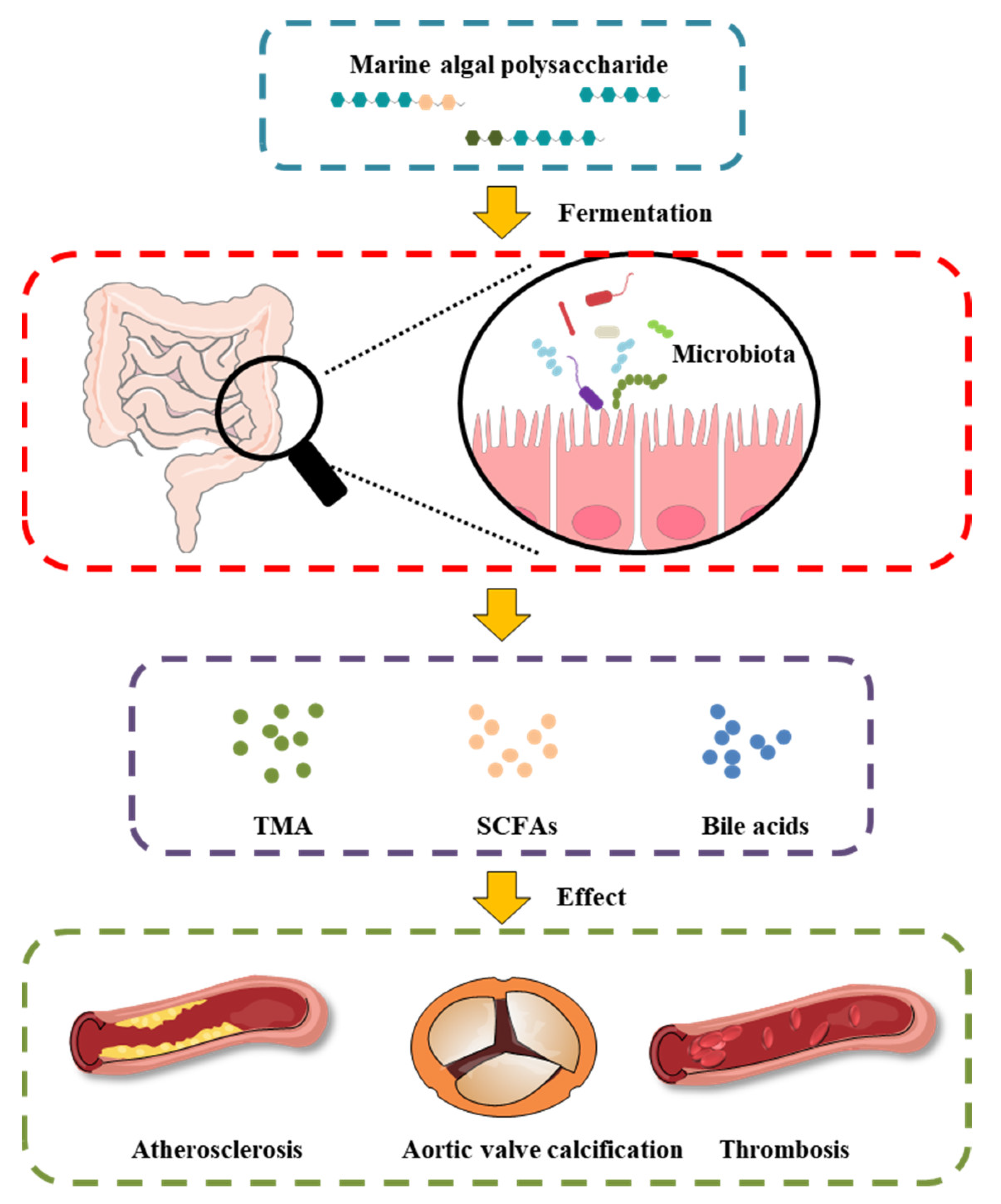
Figure 1. Effect of MAP on the intestinal microbiota and gut-derived metabolites in CVD.
This entry is adapted from the peer-reviewed paper 10.3390/foods11223550
References
- Ma, L.Y.; Chen, W.W.; Gao, R.L.; Liu, L.S.; Zhu, M.L.; Wang, Y.J.; Wu, Z.S.; Li, H.J.; Gu, D.F.; Yang, Y.J.; et al. China cardiovascular diseases report 2018: An updated summary. J. Geriatr. Cardiol. 2020, 17, 1–8.
- Celermajer, D.S.; Chow, C.K.; Marijon, E.; Anstey, N.M.; Woo, K.S. Cardiovascular Disease in the Developing World. J. Am. Coll. Cardiol. 2012, 60, 1207–1216.
- Mainous, A.G., III; Tanner, R.J.; Jo, A.; Park, K.; De Rochars, V.M.B. Trends in Cardiovascular Disease Risk in the US, 1999–2014. Am. J. Prev. Med. 2018, 55, 384–388.
- Moonesinghe, R.; Yang, Q.; Zhang, Z.; Khoury, M.J. Prevalence and Cardiovascular Health Impact of Family History of Premature Heart Disease in the United States: Analysis of the National Health and Nutrition Examination Survey, 2007–2201. J. Am. Heart Assoc. 2019, 8, e012364.
- Olinic, D.-M.; Spinu, M.; Olinic, M.; Homorodean, C.; Tataru, D.-A.; Liew, A.; Schernthaner, G.-H.; Stanek, A.; Fowkes, G.; Catalano, M. Epidemiology of peripheral artery disease in Europe: VAS Educational Paper. Int. Angiol. 2018, 37, 327–334.
- Chen, Y.; Freedman, N.D.; Albert, P.S.; Huxley, R.R.; Shiels, M.S.; Withrow, D.R.; Spillane, S.; Powell-Wiley, T.M.; Berrington de González, A. Association of cardiovascular disease with premature mortality in the United States. JAMA Cardiol. 2019, 4, 1230–1238.
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023.
- Costello, E.K.; Gordon, J.I.; Secor, S.M.; Knight, R. Postprandial remodeling of the gut microbiota in Burmese pythons. ISME J. 2010, 4, 1375–1385.
- Brown, E.M.; Allsopp, P.J.; Magee, P.J.; Gill, C.I.; Nitecki, S.; Strain, C.R.; McSorley, E.M. Seaweed and human health. Nutr. Rev. 2014, 72, 205–216.
- Xu, S.-Y.; Huang, X.; Cheong, K.-L. Recent advances in marine algae polysaccharides: Isolation, structure, and activities. Mar. Drugs 2017, 15, 388.
- Xie, X.-T.; Cheong, K.-L. Recent advances in marine algae oligosaccharides: Structure, analysis, and potential prebiotic activities. Crit. Rev. Food Sci. Nutr. 2021, 62, 1–16.
- Manlusoc, J.K.; Hsieh, C.-L.; Hsieh, C.-Y.; Salac, E.S.; Lee, Y.-T.; Tsai, P.-W. Pharmacologic application potentials of sulfated polysaccharide from marine algae. Polymers 2019, 11, 1163.
- Shannon, E.; Abu-Ghannam, N. Antibacterial derivatives of marine algae: An overview of pharmacological mechanisms and applications. Mar. Drugs 2016, 14, 81.
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101.
- Arokiarajan, M.S.; Thirunavukkarasu, R.; Joseph, J.; Ekaterina, O.; Aruni, W. Advance research in biomedical applications on marine sulfated polysaccharide. Int. J. Biol. Macromol. 2022, 194, 870–881.
- Yao, W.; Chen, X.; Li, X.; Chang, S.; Zhao, M.; You, L. Current trends in the anti-photoaging activities and mechanisms of dietary non-starch polysaccharides from natural resources. Crit. Rev. Food Sci. Nutr. 2021, 1–15.
- Zheng, L.-X.; Chen, X.-Q.; Cheong, K.-L. Current trends in marine algae polysaccharides: The digestive tract, microbial catabolism, and prebiotic potential. Int. J. Biol. Macromol. 2020, 151, 344–354.
- Ma, J.; Li, H. The role of gut microbiota in atherosclerosis and hypertension. Front. Pharmacol. 2018, 9, 1082.
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The firmicutes/bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474.
- Zhang, X.; Aweya, J.J.; Huang, Z.-X.; Kang, Z.-Y.; Bai, Z.-H.; Li, K.-H.; He, X.-T.; Liu, Y.; Chen, X.-Q.; Cheong, K.-L. In vitro fermentation of Gracilaria lemaneiformis sulfated polysaccharides and its agaro-oligosaccharides by human fecal inocula and its impact on microbiota. Carbohydr. Polym. 2020, 234, 115894.
- Wu, D.-T.; An, L.-Y.; Liu, W.; Hu, Y.-C.; Wang, S.-P.; Zou, L. In vitro fecal fermentation properties of polysaccharides from Tremella fuciformis and related modulation effects on gut microbiota. Food Res. Int. 2022, 156, 111185.
- Wu, D.-T.; He, Y.; Yuan, Q.; Wang, S.; Gan, R.-Y.; Hu, Y.-C.; Zou, L. Effects of molecular weight and degree of branching on microbial fermentation characteristics of okra pectic-polysaccharide and its selective impact on gut microbial composition. Food Hydrocolloid. 2022, 132, 107897.
- Nguyen, S.G.; Kim, J.; Guevarra, R.B.; Lee, J.-H.; Kim, E.; Kim, S.-i.; Unno, T. Laminarin favorably modulates gut microbiota in mice fed a high-fat diet. Food Funct. 2016, 7, 4193–4201.
- Grigor’eva, I.N. Gallstone disease, obesity and the Firmicutes/Bacteroidetes ratio as a possible biomarker of gut dysbiosis. J. Pers. Med. 2021, 11, 13.
- Yuan, D.; Li, C.; You, L.; Dong, H.; Fu, X. Changes of digestive and fermentation properties of Sargassum pallidum polysaccharide after ultrasonic degradation and its impacts on gut microbiota. Int. J. Biol. Macromol. 2020, 164, 1443–1450.
- Li, X.; Gao, X.; Zhang, H.; Liu, Y.; Sarker, M.M.R.; Wu, Y.; Chen, X.; Zhao, C. The anti-hyperuricemic effects of green alga Enteromorpha prolifera polysaccharide via regulation of the uric acid transporters in vivo. Food Chem. Toxicol. 2021, 158, 112630.
- Schneeberger, M.; Everard, A.; Gómez-Valadés, A.G.; Matamoros, S.; Ramírez, S.; Delzenne, N.M.; Gomis, R.; Claret, M.; Cani, P.D. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci. Rep. 2015, 5, 16643.
- Shang, Q.; Wang, Y.; Pan, L.; Niu, Q.; Li, C.; Jiang, H.; Cai, C.; Hao, J.; Li, G.; Yu, G. Dietary polysaccharide from Enteromorpha clathrata modulates gut microbiota and promotes the growth of Akkermansia muciniphila, Bifidobacterium spp. and Lactobacillus spp. Mar. Drugs 2018, 16, 167.
- Shang, Q.; Song, G.; Zhang, M.; Shi, J.; Xu, C.; Hao, J.; Li, G.; Yu, G. Dietary fucoidan improves metabolic syndrome in association with increased Akkermansia population in the gut microbiota of high-fat diet-fed mice. J. Funct. Food. 2017, 28, 138–146.
- Nowak, A.; Paliwoda, A.; Błasiak, J. Anti-proliferative, pro-apoptotic and anti-oxidative activity of Lactobacillus and Bifidobacterium strains: A review of mechanisms and therapeutic perspectives. Crit. Rev. Food Sci. Nutr. 2019, 59, 3456–3467.
- Miremadi, F.; Ayyash, M.; Sherkat, F.; Stojanovska, L. Cholesterol reduction mechanisms and fatty acid composition of cellular membranes of probiotic Lactobacilli and Bifidobacteria. J. Funct. Food. 2014, 9, 295–305.
- Wang, Y.; Li, L.; Ye, C.; Yuan, J.; Qin, S. Alginate oligosaccharide improves lipid metabolism and inflammation by modulating gut microbiota in high-fat diet fed mice. Appl. Microbiol. Biotechnol. 2020, 104, 3541–3554.
- Li, Z.-R.; Jia, R.-B.; Luo, D.; Lin, L.; Zheng, Q.; Zhao, M. The positive effects and underlying mechanisms of Undaria pinnatifida polysaccharides on type 2 diabetes mellitus in rats. Food Funct. 2021, 12, 11898–11912.
- Liu, J.; Wu, S.; Cheng, Y.; Liu, Q.; Su, L.; Yang, Y.; Zhang, X.; Wu, M.; Choi, J.-I.; Tong, H. Sargassum fusiforme alginate relieves hyperglycemia and modulates intestinal microbiota and metabolites in type 2 diabetic mice. Nutrients 2021, 13, 2887.
- Chin, Y.X.; Mi, Y.; Cao, W.X.; Lim, P.E.; Xue, C.H.; Tang, Q.J. A pilot study on anti-obesity mechanisms of Kappaphycus alvarezii: The role of native κ-carrageenan and the leftover sans-carrageenan fraction. Nutrients 2019, 11, 1133.
- Wei, B.; Zhang, B.; Du, A.-Q.; Zhou, Z.-Y.; Lu, D.-Z.; Zhu, Z.-H.; Ke, S.-Z.; Wang, S.-J.; Yu, Y.-L.; Chen, J.-W.; et al. Saccharina japonica fucan suppresses high fat diet-induced obesity and enriches fucoidan-degrading gut bacteria. Carbohydr. Polym. 2022, 290, 119411.
- Zhang, X.; You, Y.; Wang, L.; Ai, C.; Huang, L.; Wang, S.; Wang, Z.; Song, S.; Zhu, B. Anti-obesity effects of Laminaria japonica fucoidan in high-fat diet-fed mice vary with the gut microbiota structure. Food Funct. 2022, 13, 6259–6270.
- Wu, Q.; Wu, S.; Cheng, Y.; Zhang, Z.; Mao, G.; Li, S.; Yang, Y.; Zhang, X.; Wu, M.; Tong, H. Sargassum fusiforme fucoidan modifies gut microbiota and intestinal metabolites during alleviation of hyperglycemia in type 2 diabetic mice. Food Funct. 2021, 12, 3572–3585.
- Zuo, J.; Zhang, Y.; Wu, Y.; Liu, J.; Wu, Q.; Shen, Y.; Jin, L.; Wu, M.; Ma, Z.; Tong, H. Sargassum fusiforme fucoidan ameliorates diet-induced obesity through enhancing thermogenesis of adipose tissues and modulating gut microbiota. Int. J. Biol. Macromol. 2022, 216, 728–740.
- Wang, X.; Dong, J.; Liang, W.; Fang, Y.; Liang, M.; Xu, L.; Sun, W.; Li, X. Porphyran from Porphyra haitanensis alleviate obesity by reducing lipid accumulation and modulating gut microbiota homeostasis. Front. Pharmacol. 2022, 2600.
- Cheng, X.; Jiang, J.; Li, C.; Xue, C.; Kong, B.; Chang, Y.; Tang, Q. The compound enzymatic hydrolysate of Neoporphyra haitanensis improved hyperglycemia and regulated the gut microbiome in high-fat diet-fed mice. Food Funct. 2022, 13, 6777–6791.
- Liu, X.-Y.; Liu, D.; Lin, G.-P.; Wu, Y.-J.; Gao, L.-Y.; Ai, C.; Huang, Y.-F.; Wang, M.-F.; El-Seedi, H.R.; Chen, X.-H.; et al. Anti-ageing and antioxidant effects of sulfate oligosaccharides from green algae Ulva lactuca and Enteromorpha prolifera in SAMP8 mice. Int. J. Biol. Macromol. 2019, 139, 342–351.
- Li, X.; Chen, Y.; Gao, X.; Wu, Y.; El-Seedi, H.R.; Cao, Y.; Zhao, C. Antihyperuricemic effect of green alga Ulva lactuca ulvan through regulating urate transporters. J. Agric. Food. Chem. 2021, 69, 11225–11235.
This entry is offline, you can click here to edit this entry!
