Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cardiac & Cardiovascular Systems
microRNAs (miRs) are endogenous non-coding single-stranded RNAs of approximately 20 nucleotides in length that negatively regulate post-transcriptional gene functions. Acute coronary syndromes (ACS) is a result of the interplay between coronary artery in situ thrombus formation, vulnerable plaque features such as a lipid or a necrotic core, myocardial necrosis followed by fibrosis.
- acute coronary syndromes
- atherosclerosis
- cardiomyocytes
- MicroRNA
- diagnosis
1. Introduction
microRNAs (miRs) are endogenous non-coding single-stranded RNAs of approximately 20 nucleotides in length that negatively regulate post-transcriptional gene functions [1]. Since their discovery in the later years of the 20th century, miRs have become potential genetic biomarkers, among many other markers, for atherosclerotic cardiovascular disease [2,3,4]. Atherosclerotic occlusive disease concerns a large population, carrying the highest incidence of fatal and non-fatal adverse events worldwide, including myocardial infarction (MI), ischemic stroke, renal or limb ischemia [5,6,7]. Consistently, miRs has been shown to be involved in the regulation and pathogenesis of atherosclerotic stable coronary artery disease (CAD), acute coronary syndromes (ACS), both with ST-segment (STEMI) and non-ST segment elevation myocardial infarction (NSTEMI), as well as ischemia/reperfusion (I/R) injury, left ventricular remodeling (LVR) and fibrosis following ACS.
The genetic and molecular mechanisms underlying adverse outcomes in CAD are multifactorial, and sometimes difficult to interpret for clinicians. miRs have features that make them a potential diagnostic, prognostic and therapeutic target. As they regulate gene expression at the post-transcriptional level, usually by binding to the 3′-untranslated regions of target mRNAs, leading to the inhibited translation, and/or inducing degradation of the target mRNA (Figure 1) [8]. In this mechanism, a single miR can act on several or even hundreds of mRNAs. Furthermore, circulating miRs in serum are resistant to lysis. They show huge stability properties against RNase, e.g., by hiding themselves in various microvesicles (microparticles, apoptotic bodies, etc.). Thus, miRs keep the reins on all the major physiologic and pathophysiologic processes.
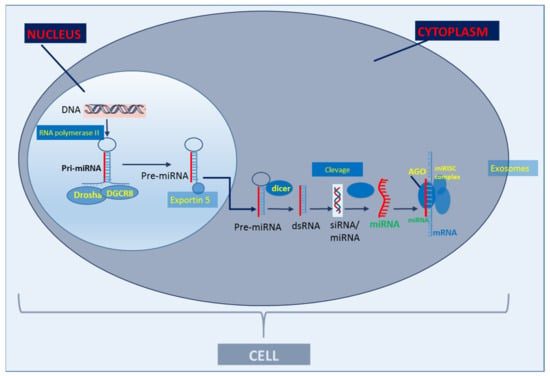
Figure 1. Basic scheme of classic miR biogenesis. The primary miRNA (Pri-miRNA) is produced in the cell nucleus through the transcription of a DNA strand mediated by RNA polymerase II. After transcription, Pri-miRNA is cleaved by the enzymatic complex DROSHA in a micro-RNA precursor (pre-miRNA). Pre-miRNA is exported to the cytoplasm by exportin-5 and cleaved by dicer (RNA degrading enzyme) and produces approximately 22 nucleotide RNA duplexes. A miRNA strand is transferred to the Argonaute complex (AGO), forming an RNA-induced silencing complex (RISC) and guides it to pair with the target messenger RNA (mRNA) through binding the miRNA seed sequence with the miRNA recognition site in the mRNA. miRs are secreted out of cells via exosomes (adapted from Creemers et al., 2012 [9]).
In a cardiovascular setting, the deprivation of vessel physiological processes, as a consequence of the degradation of responsible mRNAs, leads to the initiation of atherosclerosis [1,2,9,10,11,12]. The loss of vital mRNAs protecting against endothelial dysfunction, oxidative stress, low-grade inflammation, and many others, results in the promotion of atherosclerosis. The latter eventually leads to adverse atherosclerosis-related cardiovascular events [13,14]. Additionally, miRs play a fundamental role in either plaque destabilization or rupture, eventually triggering acute atherosclerotic ischemic events [1,2,10,11]. Eventually, they are important regulators of LVR, fibrosis, and I/R injury [10,11].
2. miRs Diagnostic in ACS
ACS is a result of the interplay between coronary artery in situ thrombus formation, vulnerable plaque features such as a lipid or a necrotic core, myocardial necrosis followed by fibrosis. As previously evidenced, several miRs follow the same kinetics as highly sensitive cardiac troponins (cTn), since they derive from myocardial necrosis [1,19].
For miRs to be considered as diagnostic markers for ACS, they must be quickly released, optimally preceding typical cardiac markers of myocardial necrosis, such as cTn, creatine kinase MB. Then, the potential miR must be characterized by high sensitivity and specificity for ACS, preferably with a power assessed with AUCs above 0.9. They should well-differentiate patients with ACS from those with stable CAD and healthy individuals (Table 2).
There is growing evidence that these criteria are fulfilled for miR-1, miR-133a and miR-133b that may have an advantage over other miRs, as their peak concentration has been documented to anticipate the peak cTn concentration, even at 2.5 h after the onset of chest pain [101]. Plasma miR-1 levels were shown to be significantly up-regulated in 93 ACS patients on admission compared to 66 healthy controls, and this decreased to similar level observed in healthy volunteers on discharge [102]. In a study by Long et al., circulating miR-1 and miR-126 in ACS patients significantly differed compared to healthy adults, with a peak change at 4 and 8 h since symptom onset, then the fold change gradually decreased over time [103]. Both miR-1 and miR-126 showed high sensitivity and specificity for ACS (Table 2). In line with this, Kazimierczyk et al. demonstrated that the concentration levels of serum miR-1 and miR-126 were higher in ACS patients on admission, compared to the controls [104]. Moreover, miR-1 correlated positively with the maximal cTn concentration (r = 0.59, p = 0.02), and negatively with the left ventricular ejection fraction (LVEF) (r = −0.76, p = 0.0004) [104]. Of note, in the work by Wang et al., and Widera et al., higher expression levels of miR-1, miR-133a, and miR-208a were found in patients with cardiac ischemia compared to healthy subjects [105,106]. In addition, Wang et al. and Zhang et al. found good accuracy for miR-499 for the early diagnosis of ACS [105,107]. Conversely, in a study by He et al., the AUC value of miR-126-3p performed better (AUC: 0.992, p < 0.001), compared to cTn (AUC 0.787, p < 0.001), and creatine kinase MB (AUC 0.863, p < 0.001) [108]. Additionally, Gidlöf et al. observed in 25 patients with STEMI an abrupt increase in miR-1, miR-133a, miR-208b and miR-499-5p with a peak within 12 h from the onset of chest pain. Moreover, expression levels of miR-208b correlated with peak cTn and the left ventricular ejection fraction [109]. In line with this, Su et al. identified miR-1 as an early marker of ACS with a similar diagnostic accuracy to cTn [110].
Xue et al. proposed a different set of miRs in the diagnosis of ACS [111]. In this study, the expression levels of plasma miR-17-5p, miR-126-5p, and miR-145-3p showed considerable diagnostic efficiency for ACS, as individual measurement, and in the combination [111]. Horvath et al. observed up-regulated levels of miR-24, miR-146a, miR-145, miR-151-3p, miR-323p, and miR-331 in STEMI compared to patients with stable CAD and healthy individuals [112]. Similarly, miR-223 and miR-191, markers of platelet activation, showed higher expression levels in patients with STEMI compared to healthy controls and stable CAD patients, indicating the presence of intracoronary thrombus as the trigger for ACS [112]. The ROC analysis confirmed the suitability of miR-331 and miR-151-3p as early biomarkers of STEMI (in a median of 2.25 hours since the onset of chest pain), while the markers of myocardial necrosis were still negative at the time of sampling [112].
Conflicting data presented by Meng et al. found decreased plasma levels of miR-143 and miR-145 in patients presenting with ACS compared to controls [113]. Both miRs were negatively correlated with Gensini score, and they showed good predictive value for the onset of ACS (miR-143: OR 0.087, 95% CI 0.026–0.384, p = 0.019, and miR-145: OR 0.179, 95% CI 0.08–0.399, p < 0.001) [113]. In line with this, data from the REGICOR registry comparing 500 samples from ACS patients matched with 500 samples from healthy controls showed that miR-143 was significantly associated with time-to-ACS (HR 0.56, 95% CI 0.38–0.82), p = 0.003) [114].
Less evidence exists for miR-23a-3p, although in a study by Bukauskas et al. miR-23a-3p showed relatively high predictive value for STEMI (AUC 0.806, 95% CI 0.694–0.917), compared to healthy individuals, and provided information on the 1-year mortality according to the GRACE and APACHE scales (p = 0.045, log-rank tests) [115]. Zhang et al. found that plasma levels of miR-21 were significantly higher in patients with AMI or angina compared to the controls. They also found a significant correlation between miR-21 and clinically established markers, including cTn and creatine kinase MB (p < 0.001) [116].
An interesting approach was presented by Kayvanpour et al. [117]. In their study, the authors developed a neural network model which incorporated 34 validated ACS miRs, showing excellent classification results with an accuracy of 0.96 (95% CI 0.96–0.97), sensitivity of 0.95, specificity of 0.96 and AUC of 0.99, compared to the one-point cTn value (accuracy of 0.89, sensitivity of 0.82, specificity of 0.96, and AUC of 0.96) [117].
Differences between STEMI and NSTEMI
Although, many studies have enrolled patient with ACS, including both STEMI and NSTEMI patients [1,19,102,103,105,106,107,108], some studies have addressed miR expression levels with respect to the type of ACS, Table 2 [100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,118,119,120,121,122,123]. Most evidence has been reported on STEMI and miRs, but much less for NSTEMI.
In a study by Bukauskas et al., higher expression levels were found for miR-23a, miR-30d, miR-146 in STEMI patients compared to healthy participants [115]. In a study by Biener et al. enrolling 137 NSTEMI patients and 905 patients admitted with chest pain (after exclusion of STEMI), higher expression levels were found for five miRs (miR-29a, miR-92a, miR-126, miR-132, and miR-133) [119]. However, the AUCs were disappointingly low, ranging between 0.577 and 0.656 for individual miRs, and 0.662 for the panel of the most predictive miRs [110]. Furthermore, in this study cTn changes had a higher predictive value for NSTEMI than miRs [110].
In a study by Liu et al. including 145 NSTEMI patients and 30 control subjects, the expression levels of miR-1, miR-133, miR-208, and miR-499 were analyzed [120]. The authors found that three out of the four analyzed miRs (miR-133, miR-208 and miR-499) demonstrated superior diagnostic accuracy than cTn (AUC: 0.778), Table 2 [120]. In contrast, Zhelankin et al. found increased plasma levels of miR-146a-5p and miR-21-5p was a general ACS circulating biomarkers and lower levels of miR-17-5p was a general biomarker of CAD [121]. In a study by Gacoń et al., the increased expression level of miR-134 in STEMI compared to NSTEMI patients was observed [122]. Interestingly in that study, patients with occluded compared with patient infarct-related coronary artery thrombosis had higher levels of miR-133a (fold change: 7.00), miR-133b (4.57), miR-34a (5.50), and miR-124 (2.55), providing a significant signature for acute plaque rupture with subsequent coronary artery thrombosis, irrespective of ACS type [122]. Thus, miR expression might indicate subjects in which a coronary angiography should be performed without further delay due to artery occlusion, and the increased risk of myocardial injury.
In 62 patients with unstable angina, Zhang et al. found that a decrease in miR-223 levels was the only independent predictor for platelet reactivity index-determined lower responders [123].
Table 2. microRNAs that are potentially diagnostic in acute coronary syndromes.
| Study Groups, N of Participants | microRNA | Down vs. Up-Regulated | Rationale for Use of Individual microRNA | AUC, or OR (95% CI), p-Value | Reference |
|---|---|---|---|---|---|
| STEMI/NSTEMI, 93 Healthy Controls, 66 |
miR-1 | Up | D, early marker, up-regulated expression, compared to healthy control group | AUC: 0.774, p < 0.001 | Ai J., 2010 [102] |
| STEMI/NSTEMI, 17 Healthy Controls, 25 |
miR-1 miR-126-3p |
Up Down |
D, early markers, changed expressions, compared to healthy control group | AUC: 0.92, p = 0.001 AUC: 0.860, p = 0.01 |
Long G., 2012 [103] |
| STEMI/NSTEMI, 33 Healthy Controls, 33 |
miR-1 miR-133a miR-208a miR-499 |
Up Up Up Up |
D, early markers, increased expressions, compared to healthy control group | AUC: 0.850, p = 0.001 AUC: 0.870, p = 0.01 AUC: 0.970, p = 0.001 AUC: 0.820, p = 0.01 |
Wang G.K., 2010 [105] |
| STEMI/NSTEMI, 142 Non-ACS chest pain, 100 Healthy Controls, 85 |
miR-499 | Up | D, early marker of ACS, 1 h after onset of chest pain, correlated with CK-MB level and cTn, but not superior to cTn (AUC: 0.90) | AUC: 0.860, p < 0.001 | Zhang L., 2015 [107] |
| STEMI/NSTEMI, 27 Healthy Controls, 30 |
miR-126-3p | Down | D, early marker, diagnostic effect superior to cTn (AUC 0.787) and CK-MB (AUC 0.863) | AUC: 0.992, p < 0.001 | He Y., 2017 [108] |
| STEMI, 25 Healthy Controls, 11 |
miR-1 miR-133a miR-208b miR-499-5p |
Up Up Up Up |
D, with a peak within 12 h from onset of chest pain, expression levels of miR-208b correlated with peak cTn and the LV ejection fraction | AUC: 0.980, p < 0.001 AUC: 0.859, p = 0.007 AUC: 1.000, p < 0.001 AUC: 0.989, p < 0.001 |
Gidlöf O.; 2011 [109] |
| STEMI, 106 NSTEMI, 68 Non-ACS chest pain, 163 |
miR-1 | Up | D, early marker of ACS within 3 h since onset of chest pain, similar AUC to cTn (AUC: 0.862, p < 0.001) | AUC: 0.863, p < 0.001 | Su T., 2020 [110] |
| STEMI, 15 NSTEMI, 14 Healthy Controls, 21 |
miR-17-5p miR-126-5p miR-145-3p | Up Up Up |
D, within 4 h after the onset of chest pain | AUC: 0.857, p < 0.001 AUC: 0.802, p < 0.001 AUC: 0.720, p = 0.01 |
Xue S., 2019 [111] |
| STEMI, 20 CAD, 20 Healthy Controls, 20 |
miR-151-3p | Up | D, proceeded release of necrotic markers, increased expression, compared to healthy controls and stable CAD | STEMI vs. controls: AUC: 0.758, p = 0.005 STEMI vs. CAD AUC: 0.754, p = 0.006 |
Horvath M., 2020 [112] |
| STEMI, 20 CAD, 20 Healthy Controls, 20 |
miR-331 | Up | D, proceeded release of necrotic markers, increased expression, compared to healthy and stable CAD | STEMI vs. controls: AUC: 0.790, p = 0.002 STEMI vs. CAD AUC: 0.773, p = 0.003 |
Horvath M., 2020 [112] |
| STEMI/NSTEMI, 78 Unstable angina, 201 Healthy Controls, 65 |
miR-143 miR-145 |
Down Down |
D, down-regulated compared to controls, good predictive value for the onset of ACS | 0.087 (0.026–0.384), p = 0.019 0.179 (0.08–0.399), p < 0.001 |
Meng L., 2022 [113] |
| ACS, 500 Healthy Controls, 500 |
miR-143 | Down | D, down-regulated compared to controls, good predictive value for the onset of ACS | 0.56 (0.38–0.82), p = 0.003 | Dégano I.R., 2020 [114] |
| STEMI, 62 Healthy Controls, 26 |
miR-23a-3p miR-30d-5p miR-146a-5p |
Down Down Down |
D, for STEMI vs. healthy controls; p, correlated with GRACE and APACHE scores of in-hospital mortality, and 1-month survival D, for STEMI vs. healthy controls D, for STEMI vs. healthy controls |
AUC: 0.806, p < 0.05 p = 0.045 (log-rank tests) AUC 0.745, p <0.05 AUC 0.800, p < 0.05 |
Bukauskas T., 2019 [115] |
| NSTEMI, 137 Chest pain *, 905 |
miR-126 miR-133 miR-134 | Up Up Up |
D, diagnostic for NSTEMI, but not superior to cTn (AUC: 0.937) | AUC: 0.578, p = 0.003 AUC: 0.656, p < 0.001 AUC: 0.506, p = 0.032 |
Biener M., 2021 [119] |
| NSTEMI, 145 Healthy Controls, 30 |
miR-1 miR-133 miR-208 miR-499 |
Up Up Up Up |
D, for NSTEMI vs. healthy controls, miR-133, miR-208 and miR-499 superior to cTn (AUC: 0.778) | AUC: 0.772, p < 0.05 AUC: 0.928, p < 0.05 AUC: 0.994, p < 0.05 AUC: 0.994, p < 0.05 |
Liu G., 2018 [120] |
| STEMI, 16 NSTEMI, 27 |
miR-134 miR-134 miR-124 miR-133b |
Up Up Up Up |
D, for STEMI, but not superior to cTn D, for occluded IRA D, for occluded IRA D, for occluded IRA |
AUC: 0.725, p = 0.002 AUC: 0.686, p = 0.016 AUC: 0.787, p < 0.001 AUC: 0.704, p = 0.006 |
Gacoń J., 2016 [122] |
| NSTEMI | miR-223-3p | Down | Marker of response to clopidogrel, targets P2Y12 receptor D, lower response to clopidogrel in NSTEMI | 0.111, (0.018–0.692), p = 0.019 | Zhang Y.Y., 2014 [123] |
* excluded patients with STEMI; ACS: acute coronary syndrome; AUC: area under the curve; CAD: coronary artery disease; CI: confidence interval; CK-MB: creatine kinase MB; cTn: cardiac troponin, D: diagnostic; IRA: infarct related artery; LV: left ventricle; miR: microRNA, NSTEMI: non-ST elevation myocardial infarction, OR: odds ratio; STEMI: ST-elevation myocardial infarction.
In summary, miR-1, miR-133, and miR-499 have the greatest potential for diagnostic biomarkers of ACS, as they are detected in blood samples before cTns. A meta-analysis of twenty-six studies enrolling in total 1973 ACS patients and 1236 healthy controls, indicated miR-1, miR-133 and miR-499 to have the highest value as diagnostic biomarkers of ACS [115]. The pooled sensitivity for miR-1 in the diagnosis of ACS was 70% (95% CI: 0.66–0.74), specificity: 81% (95% CI: 0.78–0.85), and AUC of 84%. The values for miR-133 were 82% (95%CI: 0.77–0.86), 87% (95%CI: 0.82–0.90) and 92.9% respectively, while for miR-499 were 80% (0.77–0.83), 89% (0.86–0.92) and 89.8%, respectively [124].
However, as time since ACS diagnosis to coronary artery revascularization is critical in post-myocardial injury salvage, the potential utility of these miRs may still be questionable, as quick test results are required to proceed with the treatment.
This entry is adapted from the peer-reviewed paper 10.3390/jcm11226849
This entry is offline, you can click here to edit this entry!
