Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Coatings & Films
Thermochromic smart windows are optical devices that can regulate their optical properties actively in response to external temperature changes. Due to their simple structures and as they do not require other additional energy supply devices, they have great potential in building energy-saving.
- thermochromic
- smart window
- photothermal materials
- phase change
1. Introduction
In recent years, with energy-saving and green development being vigorously promoted, smart windows have attracted much attention due to their outstanding energy-saving effect. The concept of “smart windows” was introduced by Granqvist in the 1980s [8]. They are energy-saving windows that regulate solar radiation by combining a dimming material [9,10,11] with a base material, such as glass. As optical devices, their core is the sensitive material attached to the glass. Under external excitation by light, electromagnetic radiation, and temperature, the sensitive materials will color or fade, thereby changing the windows’ colors and other optical properties so that they can automatically adjust the indoor temperature and light intensity according to the surrounding environment and ultimately achieve the purpose of saving energy consumption.
At present, most typical smart windows require an external power supply or heating device to dynamically tune the optical properties of materials in response to external stimuli. However, these additional devices have significant disadvantages, such as a complex structure, low lifespan, and high energy consumption, which also greatly hinder their commercial utilization. In contrast, solar-driven thermochromic smart windows can adjust the optical properties actively through the strong absorption of solar energy by photothermal materials, achieving a dual response to light and temperature. The features of low cost, simple structure, and low energy consumption are more conducive to large-scale applications in the future.
2. Thermochromic Materials for Smart Windows
There are many kinds of thermochromic materials that play an important role as substrates for smart windows. However, only those materials with phase change temperatures adjustable to room temperature (about 28 °C) can be used for building energy efficiency [12]. Therefore, the most widely used photothermal materials for smart windows are vanadium dioxide (VO2), thermochromic hydrogels, and liquid crystals, as shown in Figure 1.
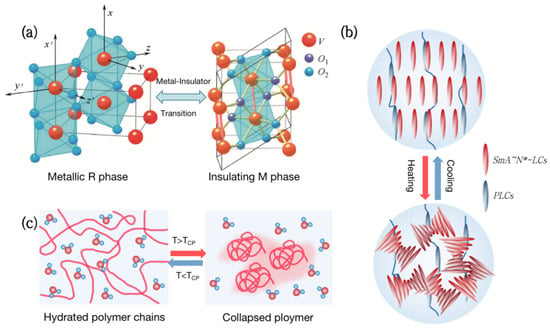
Figure 1. Types of thermochromic smart windows. (a) Metal-insulator phase transition (MIT) of vanadium dioxide (VO2). (b) Liquid crystal changes transmittance by adjusting molecular orientation in response to temperature. (c) Thermochromic hydrogels change transparency reversibly with temperature.
Vanadium dioxide (VO2) is a kind of metal-insulator phase transition (MIT) material, which is extensively utilized in thermochromic smart windows [13,14,15,16]. Its phase transition temperature is close to room temperature at 68 °C [17]. As the temperature rises, VO2 will undergo a phase transition from a semiconductor to a metal (Figure 1a), with a sudden change in infrared transmittance while keeping the visible transmittance unchanged [18]. Although VO2 films have great practical value, which are used in smart windows [19], photoelectric switches [20,21], and infrared stealth [22,23,24], the high phase transition temperature and poor optical properties are still problems to be solved.
Liquid Crystal (LC) is a special material between traditional liquids and crystalline solids. Its optical, electrical, and mechanical characteristics are anisotropic, and it can also be employed in smart windows [25,26,27,28]. From the molecular point of view, the thermochromism of Liquid Crystals can be achieved by anisotropic molecules responding to temperature and adjusting their orientation (Figure 1b). For example, the molecules inside the polymer network Liquid Crystals (PNLC) are arranged in order at low temperatures, and the refractive index of the liquid crystal and the polymer are the same, showing a highly transparent state. As the temperature rises, the liquid crystals transform into a blurred state due to the disorder of molecular orientation, and the transmittance, including near-infrared light, can change by more than 20%.
Hydrogels are both hydrophilic and hydrophobic and have been commonly employed in the field of smart windows [29,30,31,32]. Thermochromic hydrogels, such as poly(N-isopropylacrylamide) (PNIPAm) and hydroxypropyl methyl cellulose (HPMC), have a lower critical solution temperature (LCST) of around 32 °C to 40 °C. When the temperature is below the LCST, hydrophilic groups in the hydrogel form hydrogen bonds with water molecules, presenting a highly transparent state. Above the LCST, the hydrogen bonds are broken and polymers aggregate, resulting in a significant decrease in transmittance in the entire spectral range [33] (Figure 1c). Thermochromic hydrogels can reversibly change transparency with an increase in temperature, making them ideal materials for smart windows.
3. Common Photothermal Conversion Materials
Photothermal and thermochromic materials are often combined for sunlight-driven thermochromic smart windows. The strong light-absorption characteristics or localized surface plasmon resonance effects [34,35] of photothermal materials can convert solar energy into heat energy, increase the surrounding temperature, and achieve the purpose of assisting the phase transition.
3.1. Carbon-Based Nanomaterials
Carbon-based nanomaterials are a new type of photothermal material developed in recent years, such as carbon dots [36,37], graphene, and graphene oxide [38,39,40]. They have strong absorption in visible and near-infrared light and can transform solar energy into heat energy rapidly owing to their unique structures. Therefore, they show a wide range of applications in smart windows [41,42,43,44,45,46], photocatalysis [47], and tumor-targeted therapy [48].
Graphene oxide (GO) is a two-dimensional nanomaterial with a single atomic thickness obtained by the oxidation and dispersion of natural graphite. Under sunlight, most of the energy photons in visible light can be absorbed by electrons, so they are excited. When the excited-state electrons fall back to the ground state, they release heat and raise the local temperature, producing a significant photothermal effect.
3.2. Noble Metal Nanoparticles
Noble metal nanoparticles are the most researched near-infrared photothermal materials, such as Au [50,51], Ag [52], Pd [53,54], etc. Their photothermal effect is mainly derived from the strong localized surface plasmon resonance effect of nanoparticles, which is a unique phenomenon occurring in metal structures. When the frequency of the incident light matches the eigenfrequency of the free electrons in the metal, the electrons will be collectively excited and resonated. Vibrating electrons will convert kinetic energy into heat energy due to the damping effect, thus increasing the local temperature [55].
3.3. Semiconductor Nanomaterials
Due to the wide energy gap, conventional semiconductor materials need to absorb UV light with higher energy to excite the electrons and release heat in the process of falling back to the ground state. However, with the study of more kinds of semiconductor materials, it has been found that localized surface plasmon resonance (LSPR) exists not only in noble metals, but also in semiconductor materials with appreciable free carrier density, such as tin-doped indium oxide (ITO) [66,67], copper sulfide (CuxS) [68,69], titanium nitride (TiN) [70,71], etc. Compared with conventional noble metals, semiconductors can exhibit LSPR in both the ultraviolet-visible (UV-vis) and near–mid-infrared (IR) spectral regions, which significantly extends the light absorption range.
3.4. Others
Transition metal nitrides, such as titanium nitride, are versatile metal–ceramic materials with high-temperature durability, chemical stability, corrosion resistance, electrical conductivity, and also localized surface plasmon resonance properties [86,87,88]. It can replace noble metal plasmas to generate LSPR and promote material phase changes under light conditions, resulting in lower-cost thermochromic materials that are available at room temperature.
This entry is adapted from the peer-reviewed paper 10.3390/nano12213865
This entry is offline, you can click here to edit this entry!
