Type 2 diabetes mellitus (T2DM) is a growing metabolic disease characterized by insulin resistance and hyperglycemia. Current preventative and treatment strategies for T2DM and insulin resistance lack in efficacy resulting in the need for new approaches to prevent and manage/treat the disease better. In recent years, epidemiological studies have suggested that diets rich in fruits and vegetables have beneficial health effects including protection against insulin resistance and T2DM. Curcumin, a polyphenol found in turmeric, and curcuminoids have been reported to have antioxidant, anti-inflammatory, hepatoprotective, nephroprotective, neuroprotective, immunomodulatory and antidiabetic properties. Here we are summarizing the existing in vitro studies examining the antidiabetic effects of curcumin.
- insulin resistance
- diabetes
- curcumin
- curcuminoids
- skeletal muscle
- adipose
- liver
- pancreas
1. Introduction
Type 2 diabetes mellitus (T2DM) is a growing metabolic disease characterized by insulin resistance and hyperglycemia. Current preventative and treatment strategies for T2DM and insulin resistance lack in efficacy resulting in the need for new approaches to prevent and manage/treat the disease better. In recent years, epidemiological studies have suggested that diets rich in fruits and vegetables have beneficial health effects including protection against insulin resistance and T2DM. Curcumin, a polyphenol found in turmeric, and curcuminoids have been reported to have antioxidant, anti-inflammatory, hepatoprotective, nephroprotective, neuroprotective, immunomodulatory and antidiabetic properties. Here we are summarizing the existing in vitro studies examining the antidiabetic effects of curcumin.
2. Effects and Potential Applications
Overall, all available in vitro studies examining the effects of curcumin indicate increased glucose uptake and utilization by skeletal muscle cells and adipocytes, reduced hepatocyte lipid deposition, and inhibition of gluconeogenesis. Pancreatic beta cell function was improved by curcumin treatment. The figure below was created based on the in vitro studies presented in our review [1] and summarizes the main effects of curcumin.
Treatment of adipocytes with curcumin (5–100 μM) for up to 72 h resulted in reduced adipocyte differentiation, and lipid accumulation. Macrophage infiltration of adipocytes was reduced with curcumin treatment, as well as pro-inflammatory cytokine production and signaling. In addition, curcumin treatment suppressed adipogenic gene expression, while mitochondrial biogenesis and membrane potential were improved.
Treatment of hepatocytes with curcumin (5–100 μM) for up to 5 days resulted in reduced lipid deposition/lipogenic gene expression. Curcumin treatment significantly reduced inflammatory cytokine and fibrosis gene expression and increased antioxidant activities, resulting in decreased oxidative stress. In addition, gluconeogenesis was reduced, while glucokinase activity and glucose-6-phosphate levels were increased with curcumin treatment.
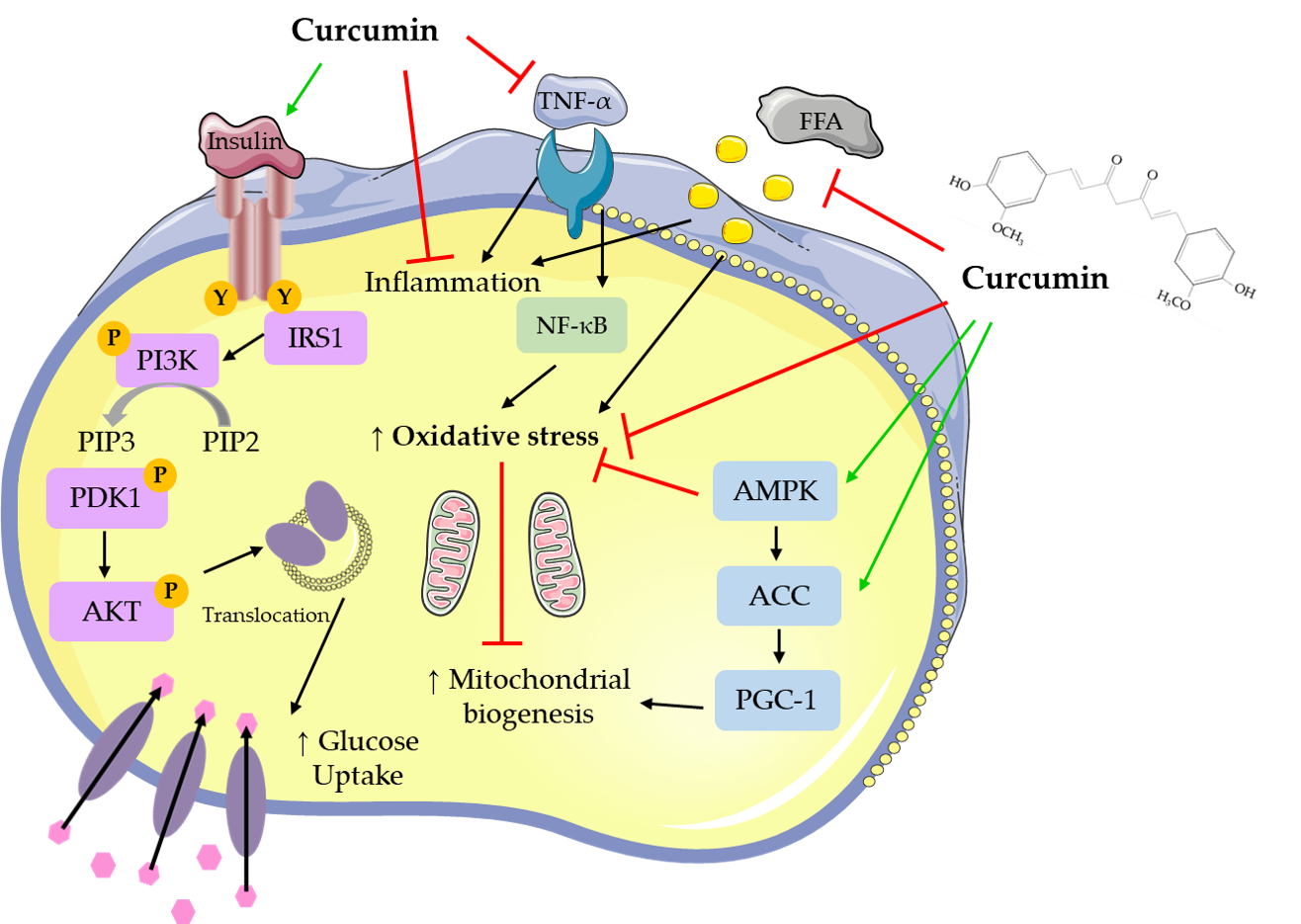
Figure 1. Cellular effects of curcumin on muscle and fat cellular signaling molecules. The figure was created based on the data of the studies [2][3][4][5][6][7][8][9][10][11][12][13][14]. AKT: protein kinase B; PIP3: phosphatidylinositol-3,4,5-triphosphate; PIP2: phosphatidylinositol 4,5-bisphosphate; ERK: extracellular signal-regulated kinase; PI3K: phosphoinositide 3-kinase; IRS1: insulin receptor substrate 1; TNF-α: tumor necrosis factor- α; AMPK: AMP-activated protein kinase; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; ACC: acetyl-CoA carboxylase; PGC-1: peroxisome proliferator-activated receptor gamma co-activator 1; FFAs: free fatty acids.
Skeletal muscle cells treated with curcumin (10–50 μM) for up to 24 h had improved glucose uptake and GLUT4 translocation. Curcumin treatment exerted anti-inflammatory effects, it reduced pro-inflammatory mRNA and cytokine levels and increased anti-inflammatory cytokine levels.
Treatment of pancreatic islets with curcumin and curcuminoids (100 pM–57 μM) for up to 24 h resulted in increased insulin secretion and islet cell recovery. HO-1 promoter activity and mRNA and protein levels and antioxidant enzyme activities were significantly increased with curcumin treatment indicating reduced apoptosis and oxidative stress.
The in vitro studies presented in our review [1] may have used different curcumin concentrations and different treatment times. A careful examination of the studies revealed that overall, the common curcumin concentrations used were in the micromolar level, with most of the studies using 10–20 μM curcumin.
Discrepancies are shown regarding curcumin’s effects on adipocytes. Studies by Green et al. (2014) [15] and Zhang et al. (2016) [16] showed that treatment of adipocytes with curcumin resulted in reduced insulin-stimulated glucose uptake and GLUT4 translocation to the plasma membrane [15][16]. These data are in contrast to other studies performed on adipocytes, demonstrating antidiabetic effects with curcumin treatment. Therefore, more studies are needed to examine in more detail the effects of curcumin on adipocytes.
Curcumin has the potential to attenuate inflammatory and oxidative stress diseases through increased antioxidant activities. A systematic and meta-analysis review by Wal et al. (2019) [17], found that curcumin blocked the oxidation process in mitochondria and reduced ROS and cytokine production and increased the activities of antioxidant enzymes [17]. Although increased antioxidant intake, such as curcumin, has been traditionally thought to result in increased health benefits [17][18][19], this concept has been recently challenged [20][21]. In the review by Halliwell (2013) [20], administration of large doses of dietary antioxidants to humans with oxidative diseases had little to no preventative or therapeutic effects. Instead, administration of weak pro-oxidants may have a greater effect on oxidative disease treatment and prevention [20]. In 2012, the United States Department of Agriculture (USDA) decided to not utilize the oxygen radical absorbance capacity (ORAC), which indicates the antioxidant power of bioactive compounds, including polyphenols such as curcumin. The decision was due to the belief that in vitro measurements of antioxidant capacity have no relevance to the effects of specific bioactive compounds on human health and that the ORAC values were routinely misused by manufacturing companies to promote their products. Clearly, the mechanisms of action of antioxidants, including curcumin, and the methodology used to quantify the effects require extensive research before human supplementation is recommended.
A search of the literature resulted in many studies focusing on the antidiabetic properties of curcumin and we have prepared two review manuscripts. The first manuscript (Antidiabetic properties of curcumin I: Evidence from in vitro studies) [1] focuses on the in vitro evidence (Nutrients, 2020;12(1)). The second manuscript (Antidiabetic properties of curcumin II: Evidence from in vivo studies) [22] focuses on the in vivo evidence (Nutrients, 2019;12(1)). Although all the available in vitro and in vivo studies suggest a strong potential of curcumin to be used in the treatment against insulin resistance and T2DM, we acknowledge the need for further clinical studies. Investigations focusing on the effective dose of curcumin in humans as well as the detailed effects on plasma glucose, lipid, insulin and HbA1c levels should be further explored.
This entry is adapted from the peer-reviewed paper 10.3390/nu12010118
References
- Danja J. Den Hartogh; Alessandra Gabriel; Evangelia Tsiani; Antidiabetic Properties of Curcumin I: Evidence from In Vitro Studies. Nutrients 2020, 12, 118, 10.3390/nu12010118.
- Minpei Kuroda; Yoshihiro Mimaki; Tozo Nishiyama; Tatsumasa Mae; Hideyuki Kishida; Misuzu Tsukagawa; Kazuma Takahashi; Teruo Kawada; Kaku Nakagawa; Mikio Kitahara; et al. Hypoglycemic effects of turmeric (Curcuma longa L. rhizomes) on genetically diabetic KK-Ay mice.. Biological & Pharmaceutical Bulletin 2005, 28, 937-939, 10.1248/bpb.28.937.
- Tozo Nishiyama; Tatsumasa Mae; Hideyuki Kishida; Misuzu Tsukagawa; Yoshihiro Mimaki; Minpei Kuroda; Yutaka Sashida; Kazuma Takahashi; Teruo Kawada; Kaku Nakagawa; et al. Curcuminoids and Sesquiterpenoids in Turmeric (Curcuma longaL.) Suppress an Increase in Blood Glucose Level in Type 2 Diabetic KK-AyMice. Journal of Agricultural and Food Chemistry 2005, 53, 959-963, 10.1021/jf0483873.
- Amanda M Gonzales; Robert A Orlando; Curcumin and resveratrol inhibit nuclear factor-kappaB-mediated cytokine expression in adipocytes. Nutrition & Metabolism 2008, 5, 17-17, 10.1186/1743-7075-5-17.
- Asma Ejaz; Dayong Wu; Paul Kwan; Mohsen Meydani; Curcumin Inhibits Adipogenesis in 3T3-L1 Adipocytes and Angiogenesis and Obesity in C57/BL Mice. The Journal of Nutrition 2009, 139, 919-925, 10.3945/jn.108.100966.
- Shao-Ling Wang; Ying Li; Ying Wen; Yan-Feng Chen; Li-Xin Na; Song-Tao Li; Chang-Hao Sun; Curcumin, a Potential Inhibitor of Up-regulation of TNF-alpha and IL-6 Induced by Palmitate in 3T3-L1 Adipocytes through NF-kappaB and JNK Pathway. Biomedical and Environmental Sciences 2009, 22, 32-39, 10.1016/s0895-3988(09)60019-2.
- Lee, Y.K.; Lee, W.S.; Hwang, J.T.; Kwon, D.Y.; Surh, Y.J.; Park, O.J. Curcumin exerts antidifferentiation effect through AMPKalpha-PPAR-gamma in 3T3-L1 adipocytes and antiproliferatory effect through AMPKalpha-COX-2 in cancer cells. J. Agric. Food Chem. 2009, 57, 305–310.
- Ariyapalli Priyanka; Sasidharan Suseela Anusree; Vijayakumar Marykutty Nisha; Kozhiparambil Gopalan Raghu; Curcumin improves hypoxia induced dysfunctions in 3T3-L1 adipocytes by protecting mitochondria and down regulating inflammation. BioFactors 2014, 40, 513-523, 10.1002/biof.1175.
- A. Priyanka; G.L. Shyni; Nair Anupama; P. Salin Raj; S.S. Anusree; K.G. Raghu; Development of insulin resistance through sprouting of inflammatory markers during hypoxia in 3T3-L1 adipocytes and amelioration with curcumin. European Journal of Pharmacology 2017, 812, 73-81, 10.1016/j.ejphar.2017.07.005.
- T Kim; J Davis; Aj Zhang; St Mathews; Auburn University Department of Nutrition and Food Science Boshell Diabetes and Metabolic Diseases Research Programs Lem Morrison Dr. Poultry Science Building Auburn AL USA; Curcumin Activates AMPK and SuppressesGluconeogenic Gene Expression in Hepatoma Cells. Planta Medica 2008, 74, , 10.1055/s-2008-1075175.
- Changkeun Kang; Euikyung Kim; Synergistic effect of curcumin and insulin on muscle cell glucose metabolism. Food and Chemical Toxicology 2010, 48, 2366-2373, 10.1016/j.fct.2010.05.073.
- Yea-Tzy Deng; Tsai-Wen Chang; Ming-Shyue Lee; Jen-Kun Lin; Suppression of Free Fatty Acid-Induced Insulin Resistance by Phytopolyphenols in C2C12 Mouse Skeletal Muscle Cells. Journal of Agricultural and Food Chemistry 2012, 60, 1059-1066, 10.1021/jf204496f.
- Asie Sadeghi; Atefeh Rostamirad; Shadisadat Seyyedebrahimi; Reza Meshkani; Curcumin ameliorates palmitate-induced inflammation in skeletal muscle cells by regulating JNK/NF-kB pathway and ROS production. Inflammopharmacology 2018, 26, 1265-1272, 10.1007/s10787-018-0466-0.
- Pratibha Chauhan; Akhilesh Kumar Tamrakar; Sunil Mahajan; G.B.K.S. Prasad; Chitosan encapsulated nanocurcumin induces GLUT-4 translocation and exhibits enhanced anti-hyperglycemic function. Life Sciences 2018, 213, 226-235, 10.1016/j.lfs.2018.10.027.
- Allan Green; Jean Krause; John M. Rumberger; Curcumin is a direct inhibitor of glucose transport in adipocytes. Phytomedicine 2014, 21, 118-122, 10.1016/j.phymed.2013.08.014.
- Deling Zhang; Yemin Zhang; Mao Ye; Youming Ding; Zhao Tang; Mingxin Li; Yu Zhou; Changhua Wang; Interference with Akt signaling pathway contributes curcumin-induced adipocyte insulin resistance. Molecular and Cellular Endocrinology 2016, 429, 1-9, 10.1016/j.mce.2016.04.013.
- Pranay Wal; Nikita Saraswat; Rashmi Saxena Pal; Ankita Wal; Madhvi Chaubey; A Detailed Insight of the Anti-inflammatory Effects of Curcumin with the Assessment of Parameters, Sources of ROS and Associated Mechanisms. Open Medicine Journal 2019, 6, 64-76, 10.2174/1874220301906010064.
- B.H. Thiers; Mortality in Randomized Trials of Antioxidant Supplements for Primary and Secondary Prevention: Systematic Review and Meta-analysis. Yearbook of Dermatology and Dermatologic Surgery 2008, 2008, 252-253, 10.1016/s0093-3619(08)70874-8.
- Katsiki, N.; Manes, C. Is there a role for supplemented antioxidants in the prevention of atherosclerosis? Clin. Nutr. 2009, 28, 3–9.
- Halliwell, B. The antioxidant paradox: Less paradoxical now? Br. J. Clin. Pharmacol. 2013, 75, 637–644.
- Zai-Qun Liu; Antioxidants may not always be beneficial to health. Nutrition 2014, 30, 131-133, 10.1016/j.nut.2013.04.006.
- Danja J. Den Hartogh; Alessandra Gabriel; Evangelia Tsiani; Antidiabetic Properties of Curcumin II: Evidence from In Vivo Studies. Nutrients 2019, 12, 58, 10.3390/nu12010058.
