1. The Hazards of Skin Glycation
The skin is mainly divided into three layers: epidermis, dermis, and subcutaneous tissue, and it is the organ with the largest contact area between the human body and the external environment. It not only protects the body from damage from the external environment and avoids the loss of water from the body but also has a certain cosmetic effect [
44]. The aging of the skin is first manifested as the aging of cells. Studies have shown that with aging, the proliferation and vitality of skin fibroblasts are reduced, leading to a decrease in the secretion of elastin fibers, collagen fibers, reticular fibers, and extracellular matrix in the dermis layer of the skin. This will result in a deepening and lengthening of skin wrinkles, pigmentation, and other manifestations of aging. A distinctive feature of aging at the molecular level is the gradual accumulation of non-enzymatically modified proteins, i.e., glycation, which produces skin problems, such as wrinkles, pigmentation, and yellowing of the skin color [
45].
1.1. The Harm of High Glucose to the Skin
Glycation is an aging reaction of naturally occurring sugars and dermal proteins [
46], which begins in early life, develops clinical symptoms at around 30, and progressively accumulates in tissues and skin due to the glycated collagens that are difficult to be decomposed [
47]. AGEs derived from natural sugars (such as glyceraldehyde-3-PO4, glucose-6-PO4, and fructose) are formed several times faster than AGEs derived from glucose. Thus, glucose is the main source of energy for mammalian cells, fueling glycolysis and the tricarboxylic acid (TCA) cycle [
48]. High-sugar foods activate the reward system of hypothalamic regulation to promote the intake of more foods that are easily metabolized as glucose [
49]. A correlation has been shown between a high-sugar diet and elevated sugar levels in the blood and skin, and a low-sugar diet can reduce skin sugar levels [
47].
In addition, the correlation between high sugar levels and skin aging can be seen in diabetic patients, where one-third of this population has skin complications [
50]. A prominent feature of aging human skin is the fragmentation of collagen fibers, which severely damages the structural integrity and mechanical properties of the skin. Elevated levels of MMP-1 and MMP-2, increased lysyl oxidase (LOX) expression, and higher crosslinked collagen in the dermis of diabetic skin lead to the accumulation of fragmented and crosslinked collagen, thereby impairing the structural integrity and mechanical properties of dermal collagen in diabetes [
51]. Collagen crosslinking makes it impossible for them to easily repair [
52], resulting in reduced skin elasticity and wrinkles [
53]. Keratinocytes and fibroblasts are the main cells involved in wound healing, but due to the high glucose (HG) microenvironment in diabetics, the functional state of these cells is impaired, thereby accelerating cellular senescence [
54].
Long-standing high glucose regulates different metabolic pathways, leading to glycotoxicity or hyperglycemic stress [
55]. These metabolic pathways include polyol pathways, glycolytic pathways, hexosamine pathways, protein kinase C (PKC) activation, and the formation of AGEs [
56]. These changes accelerate the production of ROS, increase the oxidative reaction of lipids, DNA, and proteins in various tissues [
57], and ECM disorders [
58]. High sugar also induces senescence in keratinocytes and fibroblasts [
59], upregulates p 16, p 21, and p 53 gene expression [
60], induces oxidative injury [
61], apoptosis [
62], activates transcription factor nuclear factor kappa B (NF-κB) [
63], promotes secretion of TNF-α, IL-1β, IL-6, and IL-8 [
64,
65,
66], and upregulates the expression of AGEs.
1.2. Advanced Glycaion End Products Induce Skin Aging
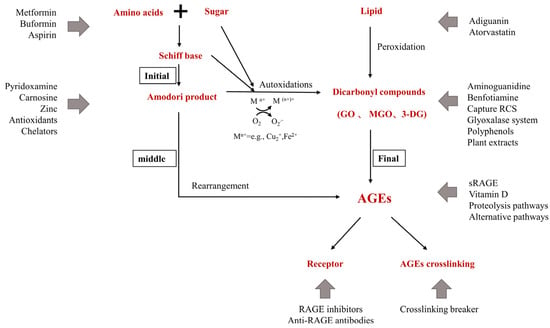
Over time, glycation in vivo causes skin AGEs to accumulate, resulting in wrinkles, loss of elasticity, dullness, and decreased function of skin, which is one of the main mechanisms of skin aging [
67]. AGEs cause pathological changes in the skin through three processes. First, AGEs interact with their specific cell receptors, altering the levels of soluble signaling molecules, such as cytokines, hormones, and free radicals. Second, in the process of non-enzymatic glycation reaction, a large number of reactive oxygen radicals are released, creating a state of oxidative stress, leading to a significantly reduced level of glutathione, VitC, and VitE in the body. This causes synthetic disorders of collagen in skin tissues. Third, AGEs alter the physical and biological properties of the original extracellular matrix proteins, such as collagen. The most important concentrations of AGEs in the skin are (from the highest to the lowest concentrations) glucosepane, CML, pentosidine, and CEL [
68]. Skin autofluorescence (SAF) has been shown to be a biomarker of cumulative skin AGEs [
69], and measuring facial fluorescence intensity allows for an assessment of the skin glycation index [
67]. SAF is also a powerful and independent predictor for cardiovascular disease and type 2 diabetes (T2D) [
70]. Most compounds on the cosmetic market focus on blocking or reversing the initial saccharification reaction, i.e., the binding between proteins and sugars, reducing the formation of early saccharification Amadori products [
71].
1.2.1. Epidermis
The epidermis is the outermost layer of the skin, which has a protective function that prevents the penetration of pathogens and regulates the body’s water loss [
72] and provides a natural “shield” against DNA damage [
73]. Keratinocytes are the main cells in the human epidermis, which rapidly differentiate into four layers after proliferation: stratum corneum, stratum granulosum, stratum spinosum, and stratum basale. Protein turnover in the epidermis is much faster, but the accumulation of AGEs can still be observed for a short term before being replaced. mRAGE expression dominates the keratinocytes of healthy human epidermis and can monitor and respond to acute and cellular responses to maintain skin homeostasis [
74]. The presence of RAGE suggests that AGE-mediated activation can have potentially negative consequences even for a short period of time. AGEs have been found to inhibit wound healing in diabetic patients by modulating the expression of MMP-9 in keratinocytes through the RAGE, ERK1/2, and p38 MAPK pathways [
75] and inducing apoptosis and inhibiting normal cell growth by activating NF-κB [
76].
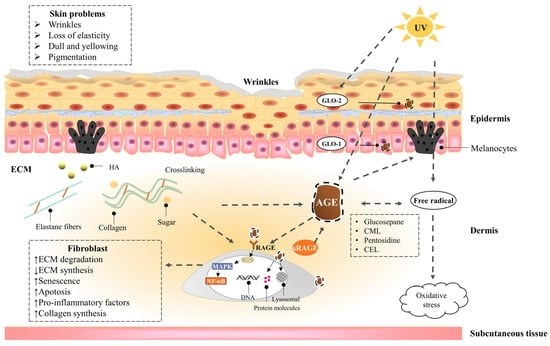
Glyoxalase is detected in the epidermis and dermis, with GLO-1 located mainly in the basal layer of the epidermis, while GLO-2 being more prominent in the upper keratinocytes. The accumulation of AGEs can be counteracted by the enzymes GLO-1 and GLO-2 of the glyoxalase system, which works synergistically to detoxify the reactive precursors of AGEs. GLO-1 and GLO-2 are more abundantly expressed in older skin. Probably as a protective mechanism, the amounts of AGEs in the basal epidermal layer of the skin are lower. Photoexposure reduces GLO-2 production and thus promotes the accumulation of AGEs [
77]. These results suggest that the glyoxalase system plays an important role in both chronological (intrinsic) aging and photoaging and acts as a defense system against skin aging [
78]. The natural vitamin B
6 analog pyridoxamine has been described as an anti-glycating agent, which not only quenches MGO but also increases GLO-1 activity. In addition, polyphenols, such as resveratrol and fisetine, can also upregulate GLO-1 expression [
79].
1.2.2. Dermis—Fibroblast
Fibroblasts are the main repair cells in the dermis but also the main cells that secrete collagen, and their normal proliferation and growth have great significance for maintaining the normal structure and physiological function of the skin. AGEs induce fibroblast senescence, matrix molecule proliferation (type I collagen, type III collagen, and type IV collagen), and metalloproteinase production (MMP1, MMP2, and MMP9) [
80]. They also promote apoptosis, reduce hyaluronic acid (HA) synthesis, and reduce elastase-type matrix metalloproteinase (ET-MMP) activity. Finally, they regulate cell dysfunction by interacting with cell membranes and accelerating the leakage of lactate dehydrogenase (LDH) from cells [
81]. AGEs modify intracellular molecules, including intermediate filament waveforms and proteasomes. The intermediate filament waveform is the main target of CML in human skin fibroblasts. DNA is also sensitive to glycation. GO causes DNA strand breaks, and MGO produces extensive DNA–protein crosslinking. RAGE levels have been found to increase over time, particularly in fibroblasts in the epidermal basal and upper dermis in elderly patients, and those interactions between AGE and RAGE lead to slower cell replication and induce a pro-inflammatory cascade [
82]. On the other hand, fibroblasts may play an important role in skin AGEs degradation and photoaging skin AGEs accumulation. AGEs can be internalized by fibroblasts through receptor-mediated endocytosis and further degraded by lysosomal proteases or proteasomes. Among them, protease D plays a major role in the degradation of intracellular AGEs [
83].
1.2.3. Dermis—Extracellular matrix (ECM)
The extracellular matrix (ECM) is a complex, non-cellular network produced primarily by fibroblasts, including proteoglycans, hyaluronic acid, adhesion glycoproteins (fibronectin and laminin), and fibrin (collagen and elastin), as well as growth factors and cytokines [
84]. They provide the mechanical strength and elastic resilience to the skin. ECM saccharification is manifested as increased skin hardness, decreased elasticity, activation of RAGE, and induction of fibroblast senescence and apoptosis [
85]. AGEs can regulate the expression of ECM proteins and alter the expression and synthesis of the enzymes responsible for their degradation. AGEs have been reported to reduce ET-MMT activity in a dose-dependent manner and have a regulatory effect on matrix metalloproteinases (MMPs) [
86]. The production of AGEs is primarily through non-enzymatic glycation of proteins in ECM. Longevity molecules are particularly susceptible to glycation, making collagen a common target for active compound modification due to its abundance and slow turnover rate. Glucosepane is the most abundant AGE that crosslinks the collagen of aging human skin. Due to its abundance, glucosepane is thought to play a major role in increasing skin stiffness and hardness. CML is one of the main AGEs in the skin and serves as an indicator of collagen glycation [
87]. Fluorescent pentosidine is a recognized marker for general AGE accumulation, and the presence of pentosidine may indicate a significant increase in the level of glucoside and other AGEs [
88]. In addition, glycated collagen induces CML expression in the dermis and epidermal compartments, resulting in an aging phenotype of poor stratification of the epidermal layer and keratinocyte cytoplasmic vacuolization [
89]. If collagen is severely crosslinked, collagenase fails to degrade the modified collagen, causing AGEs to accumulate in the dermal skin [
81].
1.3. UVA Induces Advanced Glycation End Products of the Skin
UVA exposure combined with dermal glycation are two catalysts for skin aging [
90]. The accumulation of glycation products increases with age and is amplified by ultraviolet exposure [
91]. AGEs produce superoxide anion radicals (O2
−) and hydroxyl radicals (OH▪) after UVA irradiation, increase oxidative stress in the dermal matrix [
92], damage human dermal fibroblasts, and accelerate the formation of the sugar oxidation products pentosidine and CML in actinic elastic tissues. Elastin crosslinked with AGEs cannot be degraded by elastase. UVA irradiation combined with AGEs enhances MMP1 and MMP3 mRNA expression, which induces protein expression of fibrin 1 and tropoelastin and reduces the expression of glyoxalase, which detoxifies harmful precursors of AGEs. Because glycated collagen and elastin are highly resistant to MMP degradation, a large accumulation of glycated proteins [
93] leads to skin aging and elastic tissue proliferation [
94]. In addition, keratinocytes secrete AGEs in response to UV irradiation, which stimulates melanin production through ERK and CREB signaling of RAGE, leading to skin pigmentation [
95].
Figure 1 shows the effects of UV exposure combined with AGEs on the skin.
Figure 1. The effects of UV exposure combined with AGEs on the skin. A dotted line with an arrow indicates an induced effect; a dotted line with a diamond shape indicates a suppressive effect. AGEs in the skin are endogenously generated or exogenously ingested, including CML, CEL, pentosidine, and glucosepane, etc. Collagen is more likely to be glycated due to the slow turnover rate. On the one hand, AGEs act directly on cells, leading to a decrease in cell function by activating inflammatory signaling pathways and oxidative stress through cell surface receptors, as well as by modifying cell membranes and intracellular molecules, resulting in skin problems, such as dullness, pigmentation, and wrinkles. On the other hand, AGEs crosslink with collagen and elastin in ECM and promote the secretion of melanin, causing skin problems, such as macula and loss of elasticity. In addition, UV exposure can exacerbate skin glycation by promoting the generation of AGEs, exacerbate oxidative stress, and reduce epidermal GLO-2 production, leading to the accumulation of AGEs. AGEs can be degraded by proteases through receptor-mediated fibroblast endocytosis; the glyoxalase system can detoxify the reactive precursors of AGEs; sRAGE can competitively bind to AGEs with RAGE.