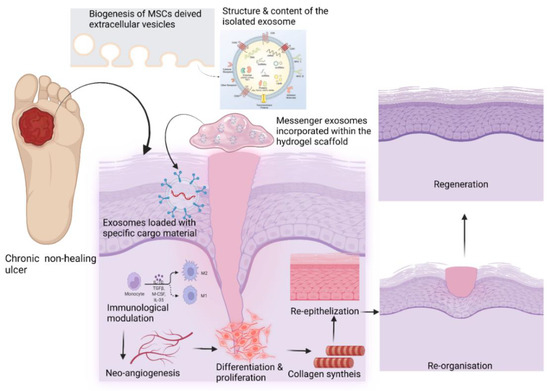
Mesenchymal stem cell-derived exosomes (MSC Exos) may favorably impact intercellular signaling and immunomodulation, promoting neoangiogenesis, collagen synthesis, and neoepithelization. Stem cell-derived EVs can build up a pro-healing environment by activating intercellular signaling, angiogenesis, proliferation, and the regional differentiation of the various cell types in tissue regeneration. In addition, the development of 3D printing technologies can help to fabricate size-specific functional scaffolds to be used in the treatment of chronic non-healing wounds. Continued advances in controlled drug delivery using MSC EVs should allow for the development of new highly effective loco-regional antibiotic delivery strategies.
1. Introduction
Extracellular vesicles (EVs) are cell-specific lipid-bound organelles that facilitate intercellular communication with their cargo elements, including proteins, nucleic acids, and certain lipids [
1]. Various types of EVs have been described, including ectosomes, microvesicles, microparticles, exosomes, oncosomes, apoptotic bodies, and exomeres [
2,
3]. Exosomes are a nanosized clinically relevant EV type with diagnostic and therapeutic applications [
4,
5,
6]. The regulated biogenesis of exosomes and the specific targeting action of cell-specific cargo materials over the recipient cells are of interest in the field of immunological disorders and regenerative medicine [
7,
8,
9].
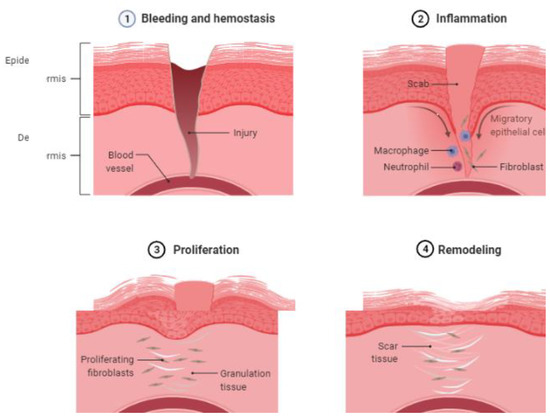
The process of wound healing has four phases: (a) hemostasis; (b) inflammatory; (c) proliferative; and (d) remodeling (
Figure 1) [
10,
11,
12,
13,
14,
15].
Figure 1. Natural course of wound healing. Four phases of wound healing (1) bleeding and hemostasis, (2) inflammation, (3) proliferation, and (4) remodeling. (created with BioRender.com).
2. Forms and Functions of Extracellular Vesicles
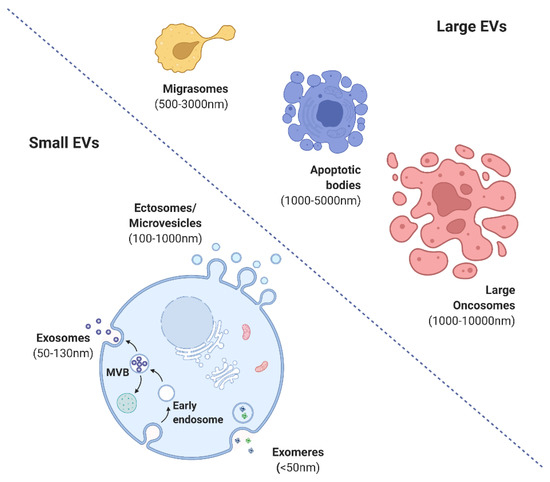
The International Society for Extracellular Vesicles (ISEV) has proposed guidelines for the nomenclature, isolation, and characterization of EVs. EVs are broadly categorized into small (exomeres (<50 mm), exosomes (<100 or 200 nm), and ectosomes (>200 nm) or shedding microvesicles (MVs)) and large (migrasomes (500–3000 nm), apoptotic bodies (1000–5000 nm), and large oncosomes (1000–10,000 nm)) (
Figure 2) [
4,
17]. Exosomes evolve by sprouting as intraluminal vesicles (ILVs) within the luminal space of late endosomes or so-called multivesicular bodies (MVBs). They are produced and released by various cells, tissues, and body fluids [
8]. EVs are involved in cell-to-cell interaction pathways with physiological and pathological functions [
18,
19]. EVs possess immunomodulatory and immunosuppressive effects and activate angiogenesis, the proliferative phase, and epithelialization.
Figure 2. Various forms of extracellular vesicles (created with BioRender.com).
3. Biogenesis of MSC-Derived EVs
In this field, the best-known mechanism is probably the endosomal sorting complex required for transport (ESCRT) [
20,
21]. The cascade promotes the activation of rho-associated protein kinase 1 (ROCK-1). ROCK-1 phosphorylates the myosin regulatory light chain and stimulates the contractile activity of actomyosin. It then leads to the formation of apoptotic bodies. Exosomes are formed during endosomal sorting. Intraluminal vesicles mature into ESCRT [
22,
23]. Microvesicle biogenesis involves the calcium-dependent enzymes calpain, gelsolin, phospholipid translocase, and scramblase [
6,
24].
The preconditioning of MSCs is performed in the first 24 h after harvesting. This period of incubation alters the cell microenvironment by inducing hypoxia, oxidative stress, and inflammation. Four cycles of ultracentrifugation are required to isolate the extracellular vesicles from the preconditioned MSCs. At the end of the first spin, the cell debris is formed; after the second spin, large-sized extracellular vesicles are collected. After the third spin, medium-sized extracellular vesicles are collected and in the last spin, small-sized extracellular vesicles are obtained [
25,
26,
27]. Various techniques are available to assess the regulation of EVs, namely: (a) a scratch wound assay (used to study cell growth and healing and especially useful to study wound closure rates and tracking wound closures for 24 h) [
28]; (b) a nanoparticle tracking analysis (performed in real time to quantify exosomes in the range of 50 to 1000 nm in a liquid suspension) [
29]; (c) dynamic light scattering (using scattered light from the Brownian motion of particles to determine the particle concentration and size) [
30]; (d) electron microscopy (scanning electron microscopy (SEM) and transmission electron microscopy (TEM) can be used to analyze the morphology of exosomes) [
31]; (e) tunable resistive pulse sensing (tRPS) (fluid is divided into two halves by a non-conductive nanomembrane. One half contains a suspension and the other half contains a particle-free electrolyte. An electric potential is applied and a resistive pulse is generated. The length of the pulse is proportional to the particle size) [
32]; and (f) cell number recovery (CNR) (the ratio of cells in the wound region at time t to cells in the wound region at time 0) [
33,
34].
4. Molecular Signaling Targets of EVs in Wound Healing
4.1. EVs in Hemostasis through Glycoproteins and Oxidases
Platelet-derived extracellular vesicles are most abundant in the circulation and help to activate platelets and the formation of fibrin clots. Platelet-derived extracellular vesicles activate both extrinsic and intrinsic pathways [
40]. They indirectly exert procoagulant effects by binding P-selectin to P-selectin glycoprotein ligand-1 (PSGL1) [
40]. Platelet-derived extracellular vesicles can also interact with NADPH oxidase (NOX) [
41]. They are involved in superoxide generation and enhance fibrin binding. Platelet-derived extracellular vesicles also induce platelet activation by collagen receptors [
42,
43]. UC-MSC-derived EVs suppress ROS-induced apoptosis through the suppression of AIF nuclear translocation and PARP-1 activation [
44].
4.2. EVs in Inflammation through Adhesion Molecules and ROS Products
Neutrophil-derived extracellular vesicles (NDEVs) show anti-inflammatory and proinflammatory functions, depending on environmental factors [
45,
46]. They increase the expression of adhesion molecules such as E-selectin and VCAM 1 and increase ROS production. NDEVs mediate inflammation by producing danger signals. Endothelium-attached NDEVs induce proinflammatory genes whereas non-adherent NDEVs induce anti-inflammatory genes [
45]. During the inflammatory phase, macrophages play an important role in the transition from the inflammatory phase to the proliferative phase. Macrophage-derived EVs induce the reprogramming of macrophages from the M1 to the M2 phenotype [
47,
48]. Extracellular vesicles derived from keratinocytes from the wound edge also cause a similar phenotype change in macrophages [
49]. M2 extracellular vesicles decrease the expression of M1 marker iNOS but increase the expression of arginase, an M2 macrophage marker [
49].
The TLR4/NF-κB/STAT3/AKT regulatory signaling pathway plays a critical role in the regulation of macrophage plasticity [
50]. LPS-preconditioned UC-MSCs modify macrophage polarization for the resolution of chronic inflammation via Exos-shuttled let-7b [
51]. Macrophage reactivity, polarization, and modulation in the wound led by MSC-derived EVs are facilitated by the transfer of miRNAs such as let-7b and -181c, which results in the downregulation of proinflammatory (TNF-α and IL-1β) micromolecules and the upregulation of anti-inflammatory (TGF-β and IL-10) micromolecules [
51,
52]. Glycolysis is the source of energy for proinflammatory M1 macrophages by inhibiting mitochondrial oxidative phosphorylation and the TCA cycle whereas mitochondrial oxidative phosphorylation is the energy feeder for anti-inflammatory M2 macrophages [
53].
4.3. EVs in Proliferation and the Mechanism in Wound Healing
EVs derived from umbilical progenitor cells have proangiogenic effects [
56]. They stimulate angiogenesis through the modulation of the AKT/ERK/STAT 3 pathway, modulation of the NOTCH pathway, increased expression of miR-126, and stimulation of the WNT/beta-catenin pathway [
56]. Treg cells play a significant role in the healing of the wound bed. Tissue-resident Treg cells provide a conductive environment for proper wound healing through the amphiregulin-TGF-β cascade [
57,
58,
59,
60]. γδTreg cells secrete KGF and IGF-1 to promote the proliferation and survival of keratinocyte [
61]. The upregulation of OCT-4 and NANOG expression and the downregulation of vinculin were observed when MSCs were incubated along with MSC-derived EVs. Such a combination delays premature senescence, facilitates stemness, and enhances glycolytic metabolism in MSCs via the activation of miR-302b and HIF-1α [
62].
BM-MSC-derived Exos accelerate wound healing by targeting fibroblasts via the Akt, Erk1/2, and STAT3 signaling pathways [
63]. FGF-2, IL-6, and -8 upregulate the Erk1/2 pathway, which results in cellular proliferation, migration, and angiogenesis [
64,
65,
66] whereas Id-1, Cox-2, VEGFA, and c-myc upregulate the Erk1/2 pathway at the mRNA level [
67,
68,
69,
70]. Mouse BM-MSC-derived EVs promoted the proliferation, migration, and tube formation of in vitro endothelial cells and increased the p-AKT and p-eNOS signaling pathways to produce angiogenesis in a healing wound [
71]. BM-MSC-derived Exos lncRNA H19 promoted wound healing in diabetic foot ulcers by upregulating PTEN via miRNA-152-3p [
72]. BM-MSC-derived EVs are rich in proliferative factors (the proliferation and promotion of the viability of keratinocytes, fibroblasts, and endothelial cells) whereas AD-MSC-derived EVs are rich in proangiogenic factors (the proliferation of endothelial cells) [
73]. Enhanced vasculogenesis was observed in wound beds when hBM-MSC-derived EVs were stimulated by deferoxamine. The combination of deferoxamine and Exos activated the PI3K/AKT signaling pathway via miR-126-mediated PTEN downregulation to stimulate angiogenesis in vitro [
74]. Exos derived from atorvastatin-pretreated BM-MSCs accelerated diabetic wound repair by enhancing angiogenesis via the AKT/eNOS pathway by upregulating miR-221-3p in endothelial cells [
75]. A static magnetic field-induced BM-MSC-derived Exos promoted neovasculogenesis to enhance wound healing through miR-21-5p by targeting SPRY2 to facilitate the PI3K/AKT and ERK1/2 signaling pathways [
76].
4.4. EVs in the Remodeling of Wound Healing
EVs facilitate collagen 1 cross-linking and promote collagen gel contraction. A few components of fibrocyte-derived EVs (FDEVs) such as hsp-90 alpha and STAT-3 promote cell motility and re-epithelialization [
45,
87,
88]. FDEVs are also rich in anti-inflammatory miRNAs such as miR124a and miR125b [
89]. MSC-derived EVs enhance the re-epithelialization, neovasculogenesis, proliferation, and migration of cellular components to the injured site by increasing MMP-9, PDGF-A, VEGF-A, FGF-2, TGF-β, and EGF and modulating the NOTCH, AKT/ERK, and WNT/β-catenin signaling pathways, enhancing the production of collagen 1 and 3, fibronectin, and extracellular matrix components [
90,
91,
92,
93,
94,
95]. Human fetal dermis-bound MSC-derived Exos induce the expression of COL1, COL3, elastin, and fibronectin by activating the NOTCH pathway [
96].
TSG-6-modified BM-MSC-derived EVs suppress scar formation by suppressing SMAD2/3 signaling and by inhibiting TGF-β1, COL1, COL3, and SMA-α protein synthesis and inflammation in the wound site [
97]. A local injection of EVs improves wound healing by increasing the mRNA for COL1 and COL3 as well as the mRNA for N-cadherin and elastin [
98]. An IV injection of AD-MSC-derived EVs migrates to the wound site and spleen, promoting wound healing [
98]. In vitro fibroblasts in response to AD-MSC-derived EVs promote the proliferation and migration of fibroblasts and keratinocytes and receive signals from COL1, COL3, MMP1, FGF2, and TGF-β1 mRNAs along with the increased expression of VEGF, c-myc, MMP-9, and fibronectin [
77,
99]. The application of PI3K/AKT inhibitor Ly294002 abrogated the EV-induced effects of fibroblasts on a wound surface [
78].
UC-MSC-derived EVs suppressed TGF-β-induced myofibroblast formation in a mouse skin wound model. These EVs were enriched with miR-21, -23a, -125b, and -145, which reduced the TGF-β/SMAD2 signaling in the fibroblasts [
103]. An accelerated re-epithelization of burned skin on rats was observed with the administration of UC-MSC-derived Exos via Wnt-4 signaling [
83]. In a skin defect mouse model, UC-MSC-derived Exos inhibited myofibroblast differentiation by suppressing the TGF-β2/SMAD2 pathway through miRNAs (miR-21, -23a, -125b, and -145), which resulted in reduced fibrosis and scar formation [
103,
104]. Amniotic fluid-MSC-derived EVs inhibited and suppressed myofibroblast aggregation and ECM synthesis via the TGF-β pathway through miRNAs such as let-7-5p, -22-3p, -27a-3p, -21-5p, and -23a-3p [
105]. UC-MSC-derived Exos promoted the phosphorylation of YAP by transporting the 14-3-3
ζ protein, which inhibited WNT/β-catenin signal transduction, enhanced collagen deposition, and inhibited excess fibroblast expansion in burn wounds. Such mechanisms have improved tissue remodeling and reduced scar formation in burn wounds [
106]. MSC-derived EVs act by targeting the injured site by producing scarless re-epithelialization and decreasing cell senescence (
Figure 3) [
107,
108].
Figure 3. Role of EVs in wound healing (created with BioRender.com).
5. New Perspectives of EV-based Therapy in Wound Healing
5.1. Engineered EV Therapy
The wound healing tendency in immunocompromised conditions such as diabetes mellitus and chronic kidney disease is negatively impacted by an impaired local immunity, which leads to a prolonged inflammatory phase and poor vascularity. In such conditions, the priming and recruitment of neutrophils are compromised and innate immunity tends to be defective. In these chronic wounds, engineered EVs have the potential to improve the chemotactic response, activate the respiratory burst of the neutrophils, and facilitate neoangiogenesis and site-specific tissue differentiation, promoting healing [
114]. EV therapy yields many advantages over cell-based therapies, including immunocompatibility, no shear stress following an injectable therapy, and a non-carcinogenic growth potential. Loco-regional angiogenesis accelerates collagen synthesis and full-thickness wound healing and improves the quality of the scar formation [
115,
116]. MSC EVs can be administered via an intravenous route, a direct injection, or a topical application. Injected MSCs exert their physiological effect at the recipient site by their paracrine secretion of extracellular vesicles rather than a direct differentiation [
117].
5.2. EV-Induced Immunomodulation
Macrophages are involved in phagocytosis and the process of tissue healing. They are phenotypically classified into M1 (classically activated) and M2 (alternatively activated) macrophages. Studies have shown improved wound healing after the administration of bone marrow-derived MSCs to a wound site by promoting M2 polarization [
54]. Polarized M2 macrophages induce the secretion of chemokines such as TNF-α, IFN-γ, and IL-1 and mediate the surge of VEGF, PDGF, and TGF-β into the local environment. Thus, the correct temporal sequence of the M1 to M2 shift mediated by EVs is important in the treatment of chronic wounds.
5.3. PRP-Derived EV Therapy
The activation of the Hippo/YAP (Yes-associated protein) signal pathway is essential for the process of epidermal re-epithelization. Platelet-rich plasma contains various growth factors essential for wound healing. EVs derived from PRP show benefits for tissue regeneration by activating the YAP pathway [
118]. A PRP-derived exosomal therapy can accelerate the process of collagen deposition in wound beds. When analyzing the dose-dependent therapeutic efficacy of platelet lysate-derived exosomes, the isolated exosomes were shown to contain a higher amount of essential growth factors (βFGF, VEGF, PDGF-BB, and TGF-β1) and small RNAs compared with the donor platelets [
119].
5.4. Bioscaffolds with Functionalized EV Therapy
Most commonly, EVs are delivered via a direct injection at the desired site. However, this can impair the function because of rapid metabolic clearance. Although MSC-derived exosomes have great potential in disease treatment, issues such as rapid clearance and the maintenance of their inadequate preservation for their viability and function remain to be addressed [
120,
121]. To date, there is no effective method to retain retrieved MSC-based EVs at the wound site. Thus, tissue-engineered biocompatible scaffold constructs provide the skeletal framework for the extracellular vesicles at the desired site to exert their prolonged therapeutic effect of healing and regeneration [
122,
123]. However, many studies have recently reported that these traditional scaffolds lack the porous structure needed for cell growth, proliferation, and migration [
124,
125]. Liu et al. designed a hydrogel glue that could retain stem cell-derived exosomes (SC Exos) to enhance the chondrogenic potential at the defect area [
126]. Furthermore, they suggested that this novel acellular exosome-rich hydrogel glue (EHG) could be used as scaffold material for tissue regeneration in chronic wounds.
This entry is adapted from the peer-reviewed paper 10.3390/life12111733