Heart failure (HF) is a major public health problem worldwide, especially coronary heart disease (myocardial infarction)-induced HF with reduced ejection fraction (HFrEF), which accounts for over 50% of all HF cases. An estimated 6 million American adults have HF. As a major feature of HF, cardiac sympathetic overactivation triggers arrhythmias and sudden cardiac death, which accounts for nearly 50–60% of mortality in HF patients. Regulation of cardiac sympathetic activation is highly integrated by the regulatory circuitry at multiple levels, including afferent, central, and efferent components of the sympathetic nervous system. Much evidence has confirmed the afferent and central neural mechanisms causing sympathoexcitation in HF. The stellate ganglion is a peripheral sympathetic ganglion formed by the fusion of the 7th cervical and 1st thoracic sympathetic ganglion. As the efferent component of the sympathetic nervous system, cardiac postganglionic sympathetic neurons located in stellate ganglia provide local neural coordination independent of higher brain centers.
- arrhythmia
- autonomic nervous system
- cardiac sympathetic activation
- heart failure
- inflammation
- stellate ganglion
1. Introduction
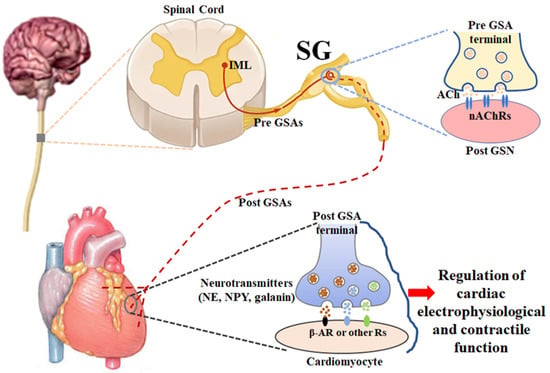
2. Anatomy and Physiology of Stellate Ganglia (Figure 1)
3. Remodeling of Cardiac Postganglionic Sympathetic Neurons and Its Role in Cardiac Sympathetic Overactivation, Malignant Arrhythmias, and Cardiac Sudden Death in HF
3.1. Structural Remodeling in Cardiac Postganglionic Sympathetic Neurons Located in Stellate Ganglia
3.2. Functional Remodeling in Cardiac Postganglionic Sympathetic Neurons Located in Stellate Ganglia
3.3. Structural Remodeling in Cardiac Postganglionic Sympathetic Nerve Terminals
This entry is adapted from the peer-reviewed paper 10.3390/ijms232113311
References
- Sweitzer, N.K. Looking ahead: Circulation: Heart failure in 2022. Circ. Heart Fail. 2022, 15, e009405.
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart disease and stroke statistics-2022 update: A report from the American Heart Association. Circulation 2022, 145, e153–e639.
- Savarese, G.; D’Amario, D. Sex differences in heart failure. Adv. Exp. Med. Biol. 2018, 1065, 529–544.
- Elgendy, I.Y.; Mahtta, D.; Pepine, C.J. Medical therapy for heart failure caused by ischemic heart disease. Circ. Res. 2019, 124, 1520–1535.
- He, J.; Ogden, L.G.; Bazzano, L.A.; Vupputuri, S.; Loria, C.; Whelton, P.K. Risk factors for congestive heart failure in US men and women: NHANESIepidemiologic follow-up study. Arch. Intern. Med. 2001, 161, 996–1002.
- Velagaleti, R.S.; Vasan, R.S. Heart failure in the twenty-first century: Is it a coronary artery disease or hypertension problem? Cardiol. Clin. 2007, 25, 487–495.
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart disease and stroke statistics-2021 update: A report from the American Heart Association. Circulation 2021, 143, e254–e743.
- Emmons-Bell, S.; Johnson, C.; Roth, G. Prevalence, incidence and survival of heart failure: A systematic review. Heart 2022, 108, 1351–1360.
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2022; cvac013, online ahead of print.
- Creager, M.A.; Faxon, D.P.; Cutler, S.S.; Kohlmann, O.; Ryan, T.J.; Gavras, H. Contribution of vasopressin to vasoconstriction in patients with congestive heart failure: Comparison with the renin-angiotensin system and the sympathetic nervous system. J. Am. Coll. Cardiol. 1986, 7, 758–765.
- Floras, J.S. Sympathetic nervous system activation in human heart failure: Clinical implications of an updated model. J. Am. Coll. Cardiol. 2009, 54, 375–385.
- Saul, J.P.; Arai, Y.; Berger, R.D.; Lilly, L.S.; Colucci, W.S.; Cohen, R.J. Assessment of autonomic regulation in chronic congestive heart failure by heart rate spectral analysis. Am. J. Cardiol. 1988, 61, 1292–1299.
- Schwartz, P.J.; De Ferrari, G.M. Sympathetic-parasympathetic interaction in health and disease: Abnormalities and relevance in heart failure. Heart Fail. Rev. 2011, 16, 101–107.
- Triposkiadis, F.; Karayannis, G.; Giamouzis, G.; Skoularigis, J.; Louridas, G.; Butler, J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J. Am. Coll. Cardiol. 2009, 54, 1747–1762.
- Du, X.J.; Cox, H.S.; Dart, A.M.; Esler, M.D. Sympathetic activation triggers ventricular arrhythmias in rat heart with chronic infarction and failure. Cardiovasc. Res. 1999, 43, 919–929.
- Gilmour, R.F. Life out of balance: The sympathetic nervous system and cardiac arrhythmias. Cardiovasc. Res. 2001, 51, 625–626.
- Kalla, M.; Herring, N.; Paterson, D.J. Cardiac sympatho-vagal balance and ventricular arrhythmia. Auton. Neurosci. 2016, 199, 29–37.
- Podrid, P.J.; Fuchs, T.; Candinas, R. Role of the sympathetic nervous system in the genesis of ventricular arrhythmia. Circulation 1990, 82, I103–I113.
- Schwartz, P.J. Cardiac sympathetic denervation to prevent life-threatening arrhythmias. Nat. Rev. Cardiol. 2014, 11, 346–353.
- Thompson, B.S. Sudden cardiac death and heart failure. AACN Adv. Crit. Care 2009, 20, 356–365.
- Tomaselli, G.F.; Zipes, D.P. What causes sudden death in heart failure? Circ. Res. 2004, 95, 754–763.
- Zhou, S.; Jung, B.C.; Tan, A.Y.; Trang, V.Q.; Gholmieh, G.; Han, S.W.; Lin, S.F.; Fishbein, M.C.; Chen, P.S.; Chen, L.S. Spontaneous stellate ganglion nerve activity and ventricular arrhythmia in a canine model of sudden death. Heart Rhythm 2008, 5, 131–139.
- Zipes, D.P. Heart-brain interactions in cardiac arrhythmias: Role of the autonomic nervous system. Cleve. Clin. J. Med. 2008, 75, S94–S96.
- Carson, P.; Anand, I.; O’Connor, C.; Jaski, B.; Steinberg, J.; Lwin, A.; Lindenfeld, J.; Ghali, J.; Barnet, J.H.; Feldman, A.M.; et al. Mode of death in advanced heart failure: The Comparison of Medical, Pacing, and Defibrillation Therapies in Heart Failure (COMPANION) trial. J. Am. Coll. Cardiol. 2005, 46, 2329–2334.
- Cygankiewicz, I.; Zareba, W.; Vazquez, R.; Vallverdu, M.; Gonzalez-Juanatey, J.R.; Valdes, M.; Almendral, J.; Cinca, J.; Caminal, P.; de Luna, A.B. Heart rate turbulence predicts all-cause mortality and sudden death in congestive heart failure patients. Heart Rhythm 2008, 5, 1095–1102.
- Doval, H.C.; Nul, D.R.; Grancelli, H.O.; Varini, S.D.; Soifer, S.; Corrado, G.; Dubner, S.; Scapin, O.; Perrone, S.V. Nonsustained ventricular tachycardia in severe heart failure. Independent marker of increased mortality due to sudden death. GESICA-GEMA Investigators. Circulation 1996, 94, 3198–3203.
- Engelstein, E.D.; Zipes, D.P. Sudden cardiac death. In Hurst’s The Heart; Alexander, R.W., Schlant, R.C., Fuster, V., Eds.; McGraw Hill: New York, NY, USA, 1998; Volume 9, pp. 1081–1112.
- Huikuri, H.V.; Castellanos, A.; Myerburg, R.J. Sudden death due to cardiac arrhythmias. N. Engl. J. Med. 2001, 345, 1473–1482.
- Jost, A.; Rauch, B.; Hochadel, M.; Winkler, R.; Schneider, S.; Jacobs, M.; Kilkowski, C.; Kilkowski, A.; Lorenz, H.; Muth, K.; et al. Beta-blocker treatment of chronic systolic heart failure improves prognosis even in patients meeting one or more exclusion criteria of the MERIT-HF study. Eur. Heart J. 2005, 26, 2689–2697.
- Maskin, C.S.; Siskind, S.J.; LeJemtel, T.H. High prevalence of nonsustained ventricular tachycardia in severe congestive heart failure. Am. Heart J. 1984, 107, 896–901.
- Podrid, P.J.; Fogel, R.I.; Fuchs, T.T. Ventricular arrhythmia in congestive heart failure. Am. J. Cardiol. 1992, 69, 82G–95G.
- Sami, M.H. Sudden death in congestive heart failure. J. Clin. Pharmacol. 1991, 31, 1081–1084.
- Singh, B.N. Significance and control of cardiac arrhythmias in patients with congestive cardiac failure. Heart Fail. Rev. 2002, 7, 285–300.
- Singh, S.N.; Carson, P.E.; Fisher, S.G. Nonsustained ventricular tachycardia in severe heart failure. Circulation 1997, 96, 3794–3795.
- Teerlink, J.R.; Jalaluddin, M.; Anderson, S.; Kukin, M.L.; Eichhorn, E.J.; Francis, G.; Packer, M.; Massie, B.M. Ambulatory ventricular arrhythmias in patients with heart failure do not specifically predict an increased risk of sudden death. PROMISE (Prospective Randomized Milrinone Survival Evaluation) Investigators. Circulation 2000, 101, 40–46.
- Al-Gobari, M.; El, K.C.; Pillon, F.; Gueyffier, F. Beta-blockers for the prevention of sudden cardiac death in heart failure patients: A meta-analysis of randomized controlled trials. BMC Cardiovasc. Disord. 2013, 13, 52.
- Babick, A.; Elimban, V.; Zieroth, S.; Dhalla, N.S. Reversal of cardiac dysfunction and subcellular alterations by metoprolol in heart failure due to myocardial infarction. J. Cell. Physiol. 2013, 228, 2063–2070.
- De Ferrari, G.M.; Schwartz, P.J. Left cardiac sympathetic denervation in patients with heart failure: A new indication for an old intervention? J. Cardiovasc. Transl. Res. 2014, 7, 338–346.
- Fiuzat, M.; Wojdyla, D.; Kitzman, D.; Fleg, J.; Keteyian, S.J.; Kraus, W.E.; Pina, I.L.; Whellan, D.; O’Connor, C.M. Relationship of beta-blocker dose with outcomes in ambulatory heart failure patients with systolic dysfunction: Results from the HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) trial. J. Am. Coll. Cardiol. 2012, 60, 208–215.
- Gheorghiade, M.; Colucci, W.S.; Swedberg, K. Beta-blockers in chronic heart failure. Circulation 2003, 107, 1570–1575.
- Nevzorov, R.; Porath, A.; Henkin, Y.; Kobal, S.L.; Jotkowitz, A.; Novack, V. Effect of beta blocker therapy on survival of patients with heart failure and preserved systolic function following hospitalization with acute decompensated heart failure. Eur. J. Intern. Med. 2012, 23, 374–378.
- Shah, R.; Assis, F.; Alugubelli, N.; Okada, D.R.; Cardoso, R.; Shivkumar, K.; Tandri, H. Cardiac sympathetic denervation for refractory ventricular arrhythmias in patients with structural heart disease: A systematic review. Heart Rhythm 2019, 16, 1499–1505.
- Vaseghi, M.; Barwad, P.; Malavassi Corrales, F.J.; Tandri, H.; Mathuria, N.; Shah, R.; Sorg, J.M.; Gima, J.; Mandal, K.; Saenz Morales, L.C.; et al. Cardiac sympathetic denervation for refractory ventricular arrhythmias. J. Am. Coll. Cardiol. 2017, 69, 3070–3080.
- Bhatt, A.S.; DeVore, A.D.; DeWald, T.A.; Swedberg, K.; Mentz, R.J. Achieving a maximally tolerated beta-blocker dose in heart failure patients: Is there room for improvement? J. Am. Coll. Cardiol. 2017, 69, 2542–2550.
- Bos, J.M.; Bos, K.M.; Johnson, J.N.; Moir, C.; Ackerman, M.J. Left cardiac sympathetic denervation in long QT syndrome: Analysis of therapeutic nonresponders. Circ. Arrhythm. Electrophysiol. 2013, 6, 705–711.
- Coleman, M.A.; Bos, J.M.; Johnson, J.N.; Owen, H.J.; Deschamps, C.; Moir, C.; Ackerman, M.J. Videoscopic left cardiac sympathetic denervation for patients with recurrent ventricular fibrillation/malignant ventricular arrhythmia syndromes besides congenital long-QT syndrome. Circ. Arrhythm. Electrophysiol. 2012, 5, 782–788.
- Moss, A.J.; Zareba, W.; Hall, W.J.; Schwartz, P.J.; Crampton, R.S.; Benhorin, J.; Vincent, G.M.; Locati, E.H.; Priori, S.G.; Napolitano, C.; et al. Effectiveness and limitations of beta-blocker therapy in congenital long-QT syndrome. Circulation 2000, 101, 616–623.
- Napolitano, C.; Priori, S.G. Diagnosis and treatment of catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 2007, 4, 675–678.
- Smith, K.V.; Dunning, J.R.; Fischer, C.M.; MacLean, T.E.; Bosque-Hamilton, J.W.; Fera, L.E.; Grant, J.Y.; Zelle, D.J.; Matta, L.; Gaziano, T.A.; et al. Evaluation of the usage and dosing of guideline-directed medical therapy for heart failure with reduced ejection fraction patients in clinical practice. J. Pharm. Pract. 2022, 35, 747–751.
- Veenis, J.F.; Rocca, H.B.; Linssen, G.C.M.; Erol-Yilmaz, A.; Pronk, A.C.B.; Engelen, D.J.M.; van Tooren, R.M.; Koornstra-Wortel, H.J.J.; de Boer, R.A.; van der Meer, P.; et al. Impact of sex-specific target dose in chronic heart failure patients with reduced ejection fraction. Eur. J. Prev. Cardiol. 2021, 28, 957–965.
- Hofferberth, S.C.; Cecchin, F.; Loberman, D.; Fynn-Thompson, F. Left thoracoscopic sympathectomy for cardiac denervation in patients with life-threatening ventricular arrhythmias. J. Thorac. Cardiovasc. Surg. 2014, 147, 404–409.
- Schneider, H.E.; Steinmetz, M.; Krause, U.; Kriebel, T.; Ruschewski, W.; Paul, T. Left cardiac sympathetic denervation for the management of life-threatening ventricular tachyarrhythmias in young patients with catecholaminergic polymorphic ventricular tachycardia and long QT syndrome. Clin. Res. Cardiol. 2013, 102, 33–42.
- Rathinam, S.; Nanjaiah, P.; Sivalingam, S.; Rajesh, P.B. Excision of sympathetic ganglia and the rami communicantes with histological confirmation offers better early and late outcomes in Video assisted thoracoscopic sympathectomy. J. Cardiothorac. Surg. 2008, 3, 50–53.
- Webster, G.; Monge, M.C. Left cardiac sympathetic denervation: Should we sweat the side effects? Circ. Arrhythm. Electrophysiol. 2015, 8, 1007–1009.
- Lathro, D.A.; Spooner, P.M. On the neural connection. J. Cardiovasc. Electrophysiol. 2001, 12, 841–844.
- Verrier, R.L.; Antzelevitch, C. Autonomic aspects of arrhythmogenesis: The enduring and the new. Curr. Opin. Cardiol. 2004, 19, 2–11.
- Zucker, I.H.; Xiao, L.; Haack, K.K. The central renin-angiotensin system and sympathetic nerve activity in chronic heart failure. Clin. Sci. 2014, 126, 695–706.
- Zucker, I.H.; Schultz, H.D.; Patel, K.P.; Wang, W.; Gao, L. Regulation of central angiotensin type 1 receptors and sympathetic outflow in heart failure. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1557–H1566.
- Zhang, D.; Liu, J.; Tu, H.; Melleman, R.L.; Cornish, K.G.; Li, Y.L. In-vivo transfection of manganese superoxide dismutase gene or NFkB shRNA in nodose ganglia improves aortic baroreceptor function in heart failure rats. Hypertension 2014, 63, 88–95.
- Wang, W.; Chen, J.S.; Zucker, I.H. Carotid sinus baroreceptor sensitivity in experimental heart failure. Circulation 1990, 81, 1959–1966.
- Schultz, H.D.; Li, Y.L.; Ding, Y. Arterial chemoreceptors and sympathetic nerve activity: Implications for hypertension and heart failure. Hypertension 2007, 50, 6–13.
- Patel, K.P.; Zheng, H. Central neural control of sympathetic nerve activity in heart failure following exercise training. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H527–H537.
- May, C.N.; Yao, S.T.; Booth, L.C.; Ramchandra, R. Cardiac sympathoexcitation in heart failure. Auton. Neurosci. 2013, 175, 76–84.
- Lymperopoulos, A.; Rengo, G.; Koch, W.J. Adrenergic nervous system in heart failure: Pathophysiology and therapy. Circ. Res. 2013, 113, 739–753.
- Felder, R.B. Mineralocorticoid receptors, inflammation and sympathetic drive in a rat model of systolic heart failure. Exp. Physiol. 2010, 95, 19–25.
- Xu, B.; Li, H. Brain mechanisms of sympathetic activation in heart failure: Roles of the renin-angiotensin system, nitric oxide and pro-inflammatory cytokines (Review). Mol. Med. Rep. 2015, 12, 7823–7829.
- Doehner, W.; Ural, D.; Haeusler, K.G.; Čelutkienė, J.; Bestetti, R.; Cavusoglu, Y.; Peña-Duque, M.A.; Glavas, D.; Iacoviello, M.; Laufs, U.; et al. Heart and brain interaction in patients with heart failure: Overview and proposal for a taxonomy. A position paper from the Study Group on Heart and Brain Interaction of the Heart Failure Association. Eur. J. Heart Fail. 2018, 20, 199–215.
- Cuevas, J. Molecular mechanisms of dysautonomia during heart failure. Focus on “Heart failure-induced changes of voltage-gated Ca2+ channels and cell excitability in rat cardiac postganglionic neurons”. Am. J. Physiol. Cell Physiol. 2014, 306, C121–C122.
- Wallis, D.; Watson, A.H.; Mo, N. Cardiac neurons of autonomic ganglia. Microsc. Res. Tech. 1996, 35, 69–79.
- Wehrwein, E.A.; Orer, H.S.; Barman, S.M. Overview of the anatomy, physiology, and pharmacology of the autonomic nervous system. Compr. Physiol. 2016, 6, 1239–1278.
- Wink, J.; van Delft, R.; Notenboom, R.G.E.; Wouters, P.F.; DeRuiter, M.C.; Plevier, J.W.M.; Jongbloed, M.R.M. Human adult cardiac autonomic innervation: Controversies in anatomical knowledge and relevance for cardiac neuromodulation. Auton. Neurosci. 2020, 227, 102674.
- Hasan, W. Autonomic cardiac innervation: Development and adult plasticity. Organogenesis 2013, 9, 176–193.
- Pardini, B.J.; Lund, D.D.; Schmid, P.G. Organization of the sympathetic postganglionic innervation of the rat heart. J. Auton. Nerv. Syst. 1989, 28, 193–201.
- Hoang, J.D.; Salavatian, S.; Yamaguchi, N.; Swid, M.A.; David, H.; Vaseghi, M. Cardiac sympathetic activation circumvents high-dose beta blocker therapy in part through release of neuropeptide Y. JCI Insight 2020, 5, e135519.
- Borovac, J.A.; D’Amario, D.; Bozic, J.; Glavas, D. Sympathetic nervous system activation and heart failure: Current state of evidence and the pathophysiology in the light of novel biomarkers. World J. Cardiol. 2020, 12, 373–408.
- de Lucia, C.; Piedepalumbo, M.; Paolisso, G.; Koch, W.J. Sympathetic nervous system in age–related cardiovascular dysfunction: Pathophysiology and therapeutic perspective. Int. J. Biochem. Cell Biol. 2019, 108, 29–33.
- Coote, J.H.; Chauhan, R.A. The sympathetic innervation of the heart: Important new insights. Auton. Neurosci. 2016, 199, 17–23.
- Meng, L.; Shivkumar, K.; Ajijola, O. Autonomic regulation and ventricular arrhythmias. Curr. Treat Options Cardiovasc. Med. 2018, 20, 38.
- Chen, P.S.; Chen, L.S.; Cao, J.M.; Sharifi, B.; Karagueuzian, H.S.; Fishbein, M.C. Sympathetic nerve sprouting, electrical remodeling and the mechanisms of sudden cardiac death. Cardiovasc. Res. 2001, 50, 409–416.
- Huang, W.A.; Boyle, N.G.; Vaseghi, M. Cardiac innervation and the autonomic nervous system in sudden cardiac death. Card. Electrophysiol. Clin. 2017, 9, 665–679.
- Kimura, K.; Ieda, M.; Fukuda, K. Development, maturation, and transdifferentiation of cardiac sympathetic nerves. Circ. Res. 2012, 110, 325–336.
- Nguyen, B.L.; Li, H.; Fishbein, M.C.; Lin, S.F.; Gaudio, C.; Chen, P.S.; Chen, L.S. Acute myocardial infarction induces bilateral stellate ganglia neural remodeling in rabbits. Cardiovasc. Pathol. 2012, 21, 143–148.
- Nakamura, K.; Ajijola, O.A.; Aliotta, E.; Armour, J.A.; Ardell, J.L.; Shivkumar, K. Pathological effects of chronic myocardial infarction on peripheral neurons mediating cardiac neurotransmission. Auton. Neurosci. 2016, 197, 34–40.
- Han, S.; Kobayashi, K.; Joung, B.; Piccirillo, G.; Maruyama, M.; Vinters, H.V.; March, K.; Lin, S.F.; Shen, C.; Fishbein, M.C.; et al. Electroanatomic remodeling of the left stellate ganglion after myocardial infarction. J. Am. Coll. Cardiol. 2012, 59, 954–961.
- Tan, A.Y.; Elharrif, K.; Cardona-Guarache, R.; Mankad, P.; Ayers, O.; Joslyn, M.; Das, A.; Kaszala, K.; Lin, S.F.; Ellenbogen, K.A.; et al. Persistent proarrhythmic neural remodeling despite recovery from premature ventricular contraction-induced cardiomyopathy. J. Am. Coll. Cardiol. 2020, 75, 1–13.
- Ajijola, O.A.; Yagishita, D.; Reddy, N.K.; Yamakawa, K.; Vaseghi, M.; Downs, A.M.; Hoover, D.B.; Ardell, J.L.; Shivkumar, K. Remodeling of stellate ganglion neurons after spatially targeted myocardial infarction: Neuropeptide and morphologic changes. Heart Rhythm 2015, 12, 1027–1035.
- Ajijola, O.A.; Wisco, J.J.; Lambert, H.W.; Mahajan, A.; Stark, E.; Fishbein, M.C.; Shivkumar, K. Extracardiac neural remodeling in humans with cardiomyopathy. Circ. Arrhythm. Electrophysiol. 2012, 5, 1010–1116.
- Ogawa, M.; Zhou, S.; Tan, A.Y.; Song, J.; Gholmieh, G.; Fishbein, M.C.; Luo, H.; Siegel, R.J.; Karagueuzian, H.S.; Chen, L.S.; et al. Left stellate ganglion and vagal nerve activity and cardiac arrhythmias in ambulatory dogs with pacing-induced congestive heart failure. J. Am. Coll. Cardiol. 2007, 50, 335–343.
- Tu, H.; Liu, J.; Zhang, D.; Zheng, H.; Patel, K.P.; Cornish, K.G.; Wang, W.Z.; Muelleman, R.L.; Li, Y.L. Heart failure-induced changes of voltage-gated Ca2+ channels and cell excitability in rat cardiac postganglionic neurons. Am. J. Physiol. Cell Physiol. 2014, 306, C132–C142.
- Augustine, G.J. How does calcium trigger neurotransmitter release? Curr. Opin. Neurobiol. 2001, 11, 320–326.
- Borst, J.G.; Sakmann, B. Calcium influx and transmitter release in a fast CNS synapse. Nature 1996, 383, 431–434.
- Zucker, R.S. Calcium and transmitter release. J. Physiol. Paris 1993, 87, 25–36.
- Tsien, R.W.; Lipscombe, D.; Madison, D.; Bley, K.; Fox, A. Reflections on Ca(2+)-channel diversity, 1988–1994. Trends Neurosci. 1995, 18, 52–54.
- Tsien, R.W.; Lipscombe, D.; Madison, D.V.; Bley, K.R.; Fox, A.P. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988, 11, 431–438.
- Benarroch, E.E. Neuronal voltage-gated calcium channels: Brief overview of their function and clinical implications in neurology. Neurology 2010, 74, 1310–1315.
- Catterall, W.A. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 2000, 16, 521–555.
- Ino, M.; Yoshinaga, T.; Wakamori, M.; Miyamoto, N.; Takahashi, E.; Sonoda, J.; Kagaya, T.; Oki, T.; Nagasu, T.; Nishizawa, Y.; et al. Functional disorders of the sympathetic nervous system in mice lacking the alpha 1B subunit (Cav 2.2) of N-type calcium channels. Proc. Natl. Acad. Sci. USA 2001, 98, 5323–5328.
- Molderings, G.J.; Likungu, J.; Gothert, M. N-Type calcium channels control sympathetic neurotransmission in human heart atrium. Circulation 2000, 101, 403–407.
- Yamada, Y.; Kinoshita, H.; Kuwahara, K.; Nakagawa, Y.; Kuwabara, Y.; Minami, T.; Yamada, C.; Shibata, J.; Nakao, K.; Cho, K.; et al. Inhibition of N-type Ca2+ channels ameliorates an imbalance in cardiac autonomic nerve activity and prevents lethal arrhythmias in mice with heart failure. Cardiovasc. Res. 2014, 104, 183–193.
- Zhang, D.; Tu, H.; Wang, C.; Cao, L.; Hu, W.; Hackfort, B.T.; Muelleman, R.L.; Wadman, M.C.; Li, Y.L. Inhibition of N-type calcium channels in cardiac sympathetic neurons attenuates ventricular arrhythmogenesis in heart failure. Cardiovasc. Res. 2021, 117, 137–148.
- Barber, M.J.; Mueller, T.M.; Henry, D.P.; Felten, S.Y.; Zipes, D.P. Transmural myocardial infarction in the dog produces sympathectomy in noninfarcted myocardium. Circulation 1983, 67, 787–796.
- Li, W.; Knowlton, D.; Van Winkle, D.M.; Habecker, B.A. Infarction alters both the distribution and noradrenergic properties of cardiac sympathetic neurons. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H2229–H2236.
- Zipes, D.P. Influence of myocardial ischemia and infarction on autonomic innervation of heart. Circulation 1990, 82, 1095–1105.
- Cao, J.M.; Fishbein, M.C.; Han, J.B.; Lai, W.W.; Lai, A.C.; Wu, T.J.; Czer, L.; Wolf, P.L.; Denton, T.A.; Shintaku, I.P.; et al. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation 2000, 101, 1960–1969.
- Oh, Y.S.; Jong, A.Y.; Kim, D.T.; Li, H.; Wang, C.; Zemljic-Harpf, A.; Ross, R.S.; Fishbein, M.C.; Chen, P.S.; Chen, L.S. Spatial distribution of nerve sprouting after myocardial infarction in mice. Heart Rhythm 2006, 3, 728–736.
- Zhou, S.; Chen, L.S.; Miyauchi, Y.; Miyauchi, M.; Kar, S.; Kangavari, S.; Fishbein, M.C.; Sharifi, B.; Chen, P.S. Mechanisms of cardiac nerve sprouting after myocardial infarction in dogs. Circ. Res. 2004, 95, 76–83.
- Kimura, K.; Kanazawa, H.; Ieda, M.; Kawaguchi-Manabe, H.; Miyake, Y.; Yagi, T.; Arai, T.; Sano, M.; Fukuda, K. Norepinephrine-induced nerve growth factor depletion causes cardiac sympathetic denervation in severe heart failure. Auton. Neurosci. 2010, 156, 27–35.
- Lorentz, C.U.; Parrish, D.C.; Alston, E.N.; Pellegrino, M.J.; Woodward, W.R.; Hempstead, B.L.; Habecker, B.A. Sympathetic denervation of peri-infarct myocardium requires the p75 neurotrophin receptor. Exp. Neurol. 2013, 249, 111–119.
- Clyburn, C.; Sepe, J.J.; Habecker, B.A. What gets on the nerves of cardiac patients? Pathophysiological changes in cardiac innervation. J. Physiol. 2022, 600, 451–461.
- Herring, N.; Kalla, M.; Paterson, D.J. The autonomic nervous system and cardiac arrhythmias: Current concepts and emerging therapies. Nat. Rev. Cardiol. 2019, 16, 707–726.
- Grkovski, M.; Zanzonico, P.B.; Modak, S.; Humm, J.L.; Narula, J.; Pandit-Taskar, N. F-18 meta-fluorobenzylguanidine PET imaging of myocardial sympathetic innervation. J. Nucl. Cardiol. 2022; online ahead of print.
- Li, J.; Zheng, L. The mechanism of cardiac sympathetic activity assessment methods: Current knowledge. Front. Cardiovasc. Med. 2022, 9, 931219.
- Orimo, S.; Yogo, M.; Nakamura, T.; Suzuki, M.; Watanabe, H. (123)I-meta-iodobenzylguanidine (MIBG) cardiac scintigraphy in α-synucleinopathies. Ageing Res. Rev. 2016, 30, 122–133.
- Rubart, M.; Zipes, D.P. Mechanisms of sudden cardiac death. J. Clin. Investig. 2005, 115, 2305–2315.
- Yokoyama, T.; Lee, J.K.; Miwa, K.; Opthof, T.; Tomoyama, S.; Nakanishi, H.; Yoshida, A.; Yasui, H.; Iida, T.; Miyagawa, S.; et al. Quantification of sympathetic hyperinnervation and denervation after myocardial infarction by three-dimensional assessment of the cardiac sympathetic network in cleared transparent murine hearts. PLoS ONE 2017, 12, e0182072.
