Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Health Care Sciences & Services
lncRNAs are master regulators of almost all biological processes. LncRNAs perform their function mostly through different chromatin-based mechanisms such as signals, decoys, guides, and scaffolds in chromatin remodelling. LncRNAs play a major role in genomic imprinting.
- long non-coding RNA (lncRNA)
- oral squamous cell carcinoma
- recurrence
1. Mechanism of Action
In last few decades, revolutionary changes in our understanding of genome regulation have emerged. The fundamental role of lncRNA is the synchronisation and regulation of gene/protein expression, thereby resulting in the fine-tuning of each and every physiological processes. Rarely, they contain short open reading frames (ORFs) [16]. The unique feature of lncRNAs is their ability to interact with other RNAs (e.g., mRNA, circRNA, miRNA, and others), DNA, and protein molecules, and consequently, they regulate many different biological processes at different levels in different ways. The formation of secondary RNA structures allows the lncRNAs to play the key role in controlling the adjacent (cis) and very distant (tans) domains in a specific chromatin region (loci) [23,41]. The functions of lncRNAs are very complex, multidimensional, and multifaceted (Figure 1). Still, they remain unclear, and current research fails to explain the sensitivity and specificity that is needed to achieve these lncRNA-mediated interactions, and the regulation of gene expressions are found to be highly cell tissue-specific in normal patients or in any patients with disease conditions like cancer.
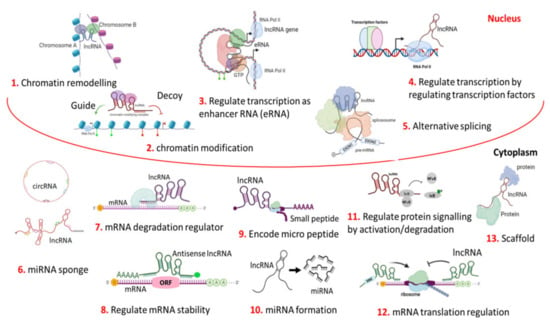
Figure 1. Different, complex, multi-dimensional functions of LncRNA. LncRNA mainly functions as signals, decoys, scaffolds, and as guides. Thus, lncRNA can act as a regulator for chromatin remodelling their environment (neighbouring or distant through intra or inter-chromosomal interaction) by positioning chromatin modifiers at the chromosomal level in the genome architecture (1). LncRNA can regulate gene expression by recruiting a chromatin-modifying complex either to activate or repress the neighbouring genes at the transcription level (2). It can also regulate transcription as an enhancer RNA or eRNA (3), or by the binding and/or activating of transcription factors to the promoter region (4). It can regulate pre-mRNA processing through the alternative splicing of mRNAs (5). In cytoplasm, at a circular form or linear form, it can sponge (silence) the function of miRNA (6). It can also act as an mRNA degradation regulator (7); mRNA stability can also be regulated by lncRNA (8). It can code for small micro peptide (9). They can generate miRNA by degrading themselves (10). It can regulate signalling cascades in different physiological pathways through the activation/deactivation of proteins in cancer cells (11). It can regulate the mRNA translation (12). Architectural scaffolding is another important function of lncRNAs. The LncRNA-mediated scaffolding of protein (RNA-protein/ribonucleoprotein) structures are called paraspeckles, which are found in interchromatin space. This is also found in several shared pathways in the cytoplasm (13).
2. LncRNA as Chromatin Regulators
LncRNAs perform their function mostly through different chromatin-based mechanisms such as signals, decoys, guides, and scaffolds in chromatin remodelling. LncRNAs play a major role in genomic imprinting. For the expression of protein coding genes, lncRNA can regulate their neighbouring (cis) or distant (trans) genomic environments by acting as an enhancer or a diffuser. LncRNA mediates epigenetic modifications by positioning chromatin-remodelling complexes to specific chromatin loci. It was estimated that 38% of lncRNA is found in various tissues binds to PRC2 and to other chromatin-modifying proteins like CoREST (REST corepressor 1 protein encoded by RCOR1 gene) and SMCX (also called JmjC-domain protein encoded by X-linked mental retardation gene SMCX or JARID1C gene) [19]. LncRNAs (ANRIL, XIST, KCNQ1OT1, and HOTAIR) bind to trithorax chromatin-activating complexes (TrxG) by recruiting different epigenetic modifiers to its assigned loci for chromatin remodelling [42,43].
ANRIL/CDKN2B, the antisense lncRNA in the INK4 locus, acts as a scaffold and transcriptionally silences the INK4b-ARF-INK4a locus. It binds to polycomb repressive complex 1 and 2 (PRC1 and PRC2) [44,45]. When PRC2 binds to the gene locus, it causes the spreading of the methylation marks, which is distinct for the transcriptional silencing of genes. A 17 Kb gene called X-inactive specific transcript (XIST), that is positioned on the human X chromosome, is responsible for the X chromosome dosage compensation. Cis-X chromosome regulation begins after the X chromosome is covered and PRC2 is recruited to its specific sites, and thus, this results in the emergence of histone H3 lysine K27 trimethylation (H3K27me3) and also causes X-linked inactivation [8,46]. LncRNA HOTAIR (HOX transcript antisense RNA) acts as a scaffold by cleaving itself to PRC2 and mediating the homeobox D cluster (HOXD) locus repression by spreading the H3K27me3 marks, and thus, this causes gene silencing [47]. HOTAIR forms many double stem-loop structures which bind to the lysine-specific demethylase1 (LSD1) and the PRC histone modification complexes [48]. KCNQ1OT1 (KCNQ1-overlapping transcript 1) antisense lncRNA, which belongs to a potassium voltage-gated channel subfamily, remains upregulated in colon cancer [49] and acts as a signal lncRNA by associating itself to G9a histone methyl-transferase and also to PRC2 [50]. When PRC2 and G9a methyl transferase are recruited to KCNQ1OT1, they mediate gene-silencing associated marks such as the demethylation of lysine 9 (H3K9me2) and lysine 27 on histone 3 [51]. KCNQ1OT1 boosts the transcriptional silencing of genes through chromatin remodelling.
3. LncRNA in Transcriptional Regulation
LncRNAs have the potential control in transcriptional regulation through modulating the expression and functions of different transcription factors, which in turn regulates different gene expression. LncRNAs can act as a co-factor of transcription factors and enzymes that are related by chromatin modification. They can regulate gene expression in cis (neighbouring) or in trans (distant) environments. Evf2 lncRNA recruits the transcriptional activator, DLX1, to the key DNA enhancer to repress the gene expression [42,52]. More recently, a detailed study on an ultra-conserved enhancer (UCE) uncovered that it has the lncRNA-dependent topological and transcriptional control, through complex effects, on the chromosome topology by interacting with multi-megabase distant genes. Evf2 lncRNA with Dlx5/6 forms a cloud-forming structure of UCE, which concurrently accomplishes the activation (Umad1, 1.6Mb distant) and repression of (Akr1b8, 27Mb distant) chr6 target genes, locally [42,52]. Recently, a new class of lncRNA, the eRNA (enhancer RNA), has been discovered at the gene enhancer region and is implicated mainly in transcriptional regulation [53].
LncRNA causes interaction with RNA-binding factors, namely heterogeneous nuclear ribonucleoproteins (hnRNPs). The hnRNPs then form ribonucleoproteins (RNPs) which then can act as enhancers to promote transcriptional processing by recruiting key transcription machinery proteins to their specific target gene promoters. RNPs can also cause the repression of gene transcription by attaching themselves to existing gene repressors. Fas and Blk are pro-apoptotic genes, for which, lncRNA mediates their repression by acting as a decoy for the transcriptional factor, NFYA [54].
LncRNAs play a major role in the modification of RNA polymerase (RNAP) II activity by interacting with the initiation complex, and guiding it to choose the precise promoter. It has been seen that in humans, the transcription of ncRNAs from the upstream region of the dihydrofolate reductase (DHFR) locus leads to the formation of a triplex in the promoter region, thus leading to the inhibition of the binding of the transcription factor, TFIID31 [55]. The basic components of RNAP II-dependent transcription machinery interactions with the lncRNA are transcribed by RNAP III. For further regulation, this lncRNA extracts their expression from an RNAP II-dependent transcription reaction. For example, the transcription of Alu elements bind to RNAP II in response to heat shock, eliminating the requirement of the pre-initiation complex and this causes repression by their domain interaction [56].
4. LncRNA in Post-Transcriptional Regulation
LncRNAs are potentially able to recognise their complimentary sequences, thus allowing for interactions that are responsible for the regulation of the post-transcriptional processing of mRNA. The processes in which lncRNAs are involved include capping, splicing, editing, transport, translation, and stability at various control sites. For example, the interaction of MALAT1 with splicing factors interrupts the process of alternate splicing. RNCR2 (also called MIAT or Gomafu) is an lncRNA that affects the mRNA splicing to provide a neuron-specific expression by interacting with the splicing factor 1 (SF1) and blocking the formation of spliceosome [50,56,57]. Natural antisense (NAT) lncRNAs recruit repressor complexes like PRC2 to the target genes and prompt the formation of RNA duplexes, inhibit cis-regulatory elements, and lead to the alternate splicing of paired genes. NAT of ZEB2 binds to the 5′splice site of an intron in 5′ UTR of ZEB2 mRNA. This intron comprises the internal ribosome entry site (IRES), which is the essential component of the translational machinery. NAT overexpression prevents splicing and increases ZEB2 expression and consequently, it down regulates the E-cadherin expression.
5. Role of lncRNA in Genomic Imprinting
Genomic imprinting is a normal epigenetic process of gene regulation by which a subset of genes can be expressed in a parent-of-origin-specific manner from one of the parental chromosomes [56]. Specific genomic loci, known as imprinting control regions (ICRs), control the genomic imprinting. Methylated and unmethylated DNA genomic imprinting regions are dependent on their parental origin for the specific expression of lncRNA genes which leads to the activation or suppression of neighbouring genes in cis-regulating machinery. Instead of PRC2, DNA methyltransferase plays a major role in lncRNA-mediated histone modification and DNA methylation in uniparental gene expression [58]. LncRNA and protein-coding genes are associated with imprinted clusters and are inversely expressed. AIRN and KCNQ1OT1 are examples of such lncRNAs that are responsible for the genomic imprinting of paternally inherited genes [59]. KCNQ1OT1 takes a crucial step in the long-range bidirectional repression of chromatin structures of different protein-coding genes by associating with the chromatin-modifying complexes, EED and G9A/EHMT2, and with the RNA itself. KCNQ1OT1/LIT1 is considered as an imprinted control region 2 (ICR2), which consists of at least eight genes that are expressed from the maternal allele [60]. KCNQ1OT1 silences the KCNQ1 imprinting control region by functioning like an organiser and by interacting with chromatin-modifying complexes, EED and G9A/EHMT2, and with the RNA itself [60,61,62,63,64]. Insulin-like growth factor-2 (Igf2) and insulin-like growth factor-2 receptor (Igf2r) are examples of maternally and paternally imprinted genes [65]. H19, an lncRNA plays an important role in regulating maternal imprinting which is essential for the regulation of cellular differentiation during embryogenesis in humans [66]. H19, after associating with methyl-CpG-binding-domain protein1 (MBD1), recruits histone-lysine-methyl transferase-containing complexes which form repressive H3K9 methylation marks on the targeted imprinting loci. The absence of H19-mediated maternal imprinting may cause Beckwith-Wiedemann Syndrome (BWS) and correlates with an increased risk of developing a Wilms tumour of the kidney [67,68,69,70]. The dysregulation of imprinting genes is reported in some pathological conditions, including cancer [71]. Collectively, the above information has revealed that the functions of lncRNAs are unique, due to their ability to establish molecular interactions with proteins and all types of nucleic acids to modulate/regulate their accessibility, localisations, and functions. The multidimensional and multifaceted functional versatility and flexibility of lncRNAs has emerged in recent days [72,73]. The complete understanding of the functional plasticity of lncRNAs will give fundamental insight into the mechanisms that lncRNAs employ for gene/protein regulation and the pathophysiological processes of cancers, including OSCC.
This entry is adapted from the peer-reviewed paper 10.3390/cancers14225590
This entry is offline, you can click here to edit this entry!
