Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
乳腺癌(BC)是一个严重的全球性挑战,抑郁症是BC的危险因素和合并症之一。最近,关于BC和抑郁症合并症的研究集中在下丘脑 - 垂体 - 肾上腺轴的功能障碍和炎症反应的持续刺激上。然而,合并症的进一步机制尚不清楚。环氧化物代谢已被证明在分散报告的共病机制中具有调节功能。因此,本文综述了环氧化物代谢在抑郁症和BC中的作用。综合综述揭示了环氧化物代谢的失衡及其与BC和抑郁症共有的下游效应,包括炎症过度表达、毒性二醇上调和脂质代谢紊乱。这些下游效应主要通过肝脏调控参与乳腺恶性肿瘤微环境的构建。
- depression
- epoxide metabolism
- comorbidity
- tumor microenvironment
- breast cancer
1. 简介
癌症是世界上死亡率最高的恶性疾病之一,其发病率继续快速增长[1,2]。女性人群中癌症相关死亡的主要原因是乳腺癌(BC)[3]。自2020年以来,BC一直是全球癌症发病率的主要原因,占所有癌症病例的11.7%[3,4]。BC也是全球癌症死亡的第五大原因[3]。此外,BC是提高每个国家预期寿命的障碍,在全球范围内造成沉重的经济负担以及健康和社会挑战[3]。BC是一种高度异质性疾病,其发展与遗传、饮食和环境因素有关[5]。各种类型的BC大致可分为激素受体状态(雌激素受体ER或孕酮受体PR)、人表皮生长因子受体状态(HER2)和三阴性状态(TNBC)[5,6,7,8]。目前的主流治疗选择包括常规化疗、单克隆抗体和全身性联合给药[5]。尽管BC诊断水平的提高多年来提高了生存率,但治疗的副作用、应激的影响以及生存质量不理想仍然引起公众的关注[9]。BC的躯体和精神合并症发生率较高,主要由慢性应激引起[10]。抑郁症作为BC的重要危险因素和合并症,几十年来一直困扰着BC女性。在缺乏对个人、家庭和专业领域的精准管理的情况下,女性BC患者处于精神压力之下,最终带来更重的身体负担。研究表明,抑郁是住院频率较高、住院时间较长、生活质量较低和治疗依从性较低的独立预测因子[11]。抑郁也被证明是晚期BC患者诊断的重要预测指标,BC患者的自杀率与抑郁表型的临床症状高度相关[12]。据报道,合并抑郁与癌症患者预后不良和死亡率增加有关[13]。一项研究表明,BC患者在癌症药物治疗期间和之后的抑郁症患病率为15%。焦虑和抑郁的治疗与化疗后神经认知功能下降和海马体积减少有关[9,14,15,16,17]。更重要的是,在当前全球传染病流行的背景下,BC患者由于工作和就业的影响,容易出现情绪障碍和认知功能障碍[18]。一项meta分析表明,负面情绪会显著增加BC发病风险[19]。因此,BC和抑郁症的合并症是一个不可避免的生物医学问题。
迄今为止,大多数关于BC和抑郁合并症的研究都集中在四个方面:炎症和氧化/亚硝化应激、免疫监测降低、自主神经系统异常激活以及HPA[20]。事实上,外周多巴胺(DA)和犬尿氨酸(KYN)的不平衡被提出来积极预测BC患者的抑郁[21]。此外,HPA和交感神经系统的持续激活被认为可以促进BC生长。不幸的是,由于信息的分散,抑郁和BC之间的桥接机制尚不清楚,因为合并症的病因和最终影响仅部分讨论。环氧化物代谢是介导炎症、肿瘤和免疫监视的重要代谢过程,主要发生在肝脏、肾脏和血管中[22]。
环氧化物代谢在BC中起显着的调节作用。可溶性环氧化物水解酶(sEH)是环氧化物代谢中必不可少的中间酶,对抑郁和BC的发病机制具有重要影响[22,23]。一些研究表明,sEH的上调与神经系统疾病密切相关[24]。在BC组织中也发现sEH水平的降低,而sEH水平的增加抑制BC增殖。其他学者表明,sEH可以通过水解有毒环氧化物来促进BC细胞增殖,这与以往的研究不一致[25,26]。因此,sEH介导的环氧化物代谢可能是研究的关键领域,也是BC和抑郁症的关键共病机制之一。然而,提出的证据是有争议的。根据这项研究,环氧化物代谢主要发生在肝脏,sEH可能对BC的不同亚型有不同的影响。此外,环氧化物代谢参与介导免疫应答和调节肿瘤微环境(TME)中的脂质稳态[27]。研究人员证实,BC合并抑郁患者的血浆白细胞介素6(IL-6)水平更高,并且还受sEH调节[28,29]。sEH介导的环氧化物代谢可能与更深层次的机制有关,这是争议的关键点。
2.抑郁是BC的重要危险因素和合并症
抑郁症是报告的癌症危险因素之一,被称为BC的合并症。研究人员发现,BC幸存者抑郁的发生率很高,治疗期间和治疗后的抑郁发生率为15%[9,30]。目前的研究强调神经激素信号系统是BC和抑郁症的主要共享机制。交感神经系统(SNS)和HPA是影响神经系统并有助于BC发育的两种应激反应[31]。当抑郁症发生时,慢性应激源会激活HPA轴,导致肾上腺素和儿茶酚胺释放。HPA轴激活后,肾上腺素激活BC-肾上腺素能受体,积累髓源性抑制细胞(MDSC),并促进BC发育[32]。肾上腺皮质分泌的皮质醇通过激活糖皮质激素受体(GR)信号通路、血清/糖皮质激素调节激酶1(SGK1)和丝裂原活化蛋白激酶磷酸酶1(MKP1)/双特异性磷酸酶1(DUSP1)来促进BC细胞发育[33]。同时,皮质醇通过抑制免疫功能,降低自然杀伤(NK)细胞活性和T细胞增殖,导致肿瘤免疫监视降低[34](图1)。
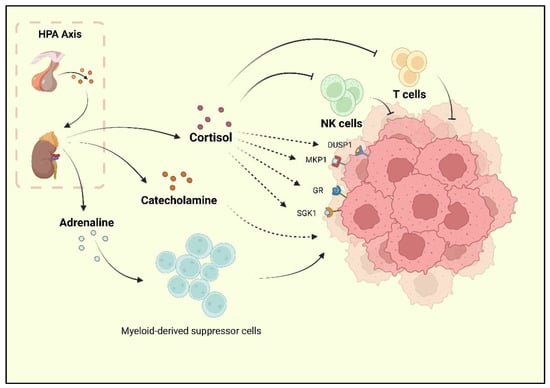
图1.据报道,由压力激活的HPA轴可促进皮质醇,肾上腺素和儿茶酚胺的释放。皮质醇可以抑制NK细胞和T细胞的活性,并促进GR,MKP1,SGK1和DUSP1的活化,这些是乳腺癌进展的积极因素。儿茶酚胺对BC也有促进作用。此外,肾上腺素可以上调MDSCs的生物活性,间接促进BC的进展。
此外,抑郁症与BC有关,部分原因是抑郁表型诱导的巨噬细胞活性增加。M1巨噬细胞是重度神经系统疾病患者炎症的重要因素[35]。重性抑郁障碍研究发现,外周血单核细胞(PBMC)中循环细胞因子水平升高,PBMC中NF-kB水平升高[36]。脂肪细胞和BC肿瘤细胞释放趋化因子(例如C-C基序趋化因子配体2(CCL2)、C-C基序趋化因子配体5(CCL-5)或集落刺激因子(CSF-1)),促进单核细胞和巨噬细胞迁移到BC微环境中[37,38]。这些巨噬细胞含有M1和M2表型,而M1巨噬细胞总是在BC微环境中转化为M2,单核细胞也是如此[39,40,41,42]。乳腺基质中的脂肪细胞是IL-10(IL-10)的重要来源,IL-10也有助于巨噬细胞极化为BC的M2表型[43,44,45]。临床研究还表明,IL-10是TNBC、ER阴性或PR阴性病例预后不良的独立因素[46,47]。
抑郁症患者长期应激诱发的慢性全身性炎症已被明确证明是致癌的起始因素[48]。IL-6是促炎细胞因子之一,是抑郁症的信号传导启动子和病理产物[49]。研究表明,高水平的IL-6与抑郁症的慢性病程有关,IL-6高表达患者的抑郁严重程度也增加。老年抑郁症患者的研究结果显示,IL-6水平高于健康老年人[50]。同样,IL-6在BC省也起着重要作用的促癌作用。临床证据表明,IL-6诱导与BC患者预后不良有关,血浆IL-6水平与病理分级呈正相关。一项临床前研究得出,IL-6/IL-6R/gp130通路促进BC的生长和转移,而抑制该通路不利于BC的发展。因此,IL-6 可能导致合并症中的 BC 和抑郁。此外,TNF-α是抑郁症中的致病细胞因子。一项研究发现,重性抑郁患者的促炎细胞因子TNF-α和IL-6水平显著升高[51]。同时,抗TNF-α药物被发现是抗抑郁药[52]。本文还讨论了TNF-α对BC的双重影响[53]。免疫应答进一步表明,慢性炎症是抑郁症和BC合并症的重要基础。激素调节和细胞因子作用的事实已被广泛提及,但合并症发病机制的中间阶段仍不清楚。
3. sEH的不同状态介导BC环氧化物代谢
sEH介导的环氧化物代谢不仅在抑郁中起作用,而且在BC微环境的形成中起作用。然而,sEH对BC的影响是有争议的。一方面,sEH导致EETs减少,这是BC的促进因素[54]。同时,14,15-EET可促进BC组织的血管生成并上调血管内皮生长因子(VEGF)[55]。一项临床研究表明,14, 15-EET诱导整合素αvβ3表达和FAK/PI3K/AKT活化,从而增强体内BC细胞(MCF-7和MDA-MB-231)的基质产生和顺铂耐药性[56]。
另一方面,细胞毒性白细胞代谢物可通过sEH降解为二醇,表明二元醇可刺激MCF-7细胞(ER+PR+HER2-)的增殖[24,57,58,59]。更严重的是,炎症性乳腺癌(IBC)被认为是BC的一种快速发展和转移类型。EET的降解导致促炎微环境的产生,这反过来又成为IBC的危险因素。因此,sEH似乎对BC有进一步的促销影响。研究人员发现,具有不同受体表型的BC具有不同水平的sEH,这意味着参与sEH的环氧化物代谢将对BC的不同亚型产生不同的影响,甚至可能相反[7,25]。sEH对BC不同表型影响的几个证据已经通过基于组学的途径分析被鉴定出来[7]。研究表明,sEH在HER2+(75%)、ER+PR+(68%)和三阳性BC(TPBC)(67%)中呈高度阳性,但表达最弱的出现在TNBC(46%)。同时,HER2+的sEH抑制作用显着降低(sEH为33%的强烈阳性),特别是与TNBC相比。TNBC保持最低的sEH表达,而CYP450,包括CYP2C8,2C9,2J2和CYP3A4,在TNBC中表现出强烈的表达,已知其促进AA,LA,DHA和EPA转化为EET,EpOMEs,EpETEs和EpDPAs。源自这些CYP450的EET已被证实可促进TNBC的侵袭和转移[54]。尽管TNBC引起对CYP450和EET的敏感反应,但TNBC的不同亚型仍显示出相反的现象[7]。据报道,TNBC 分为几种亚型,包括间充质样亚型和基底样亚型。[60,61,62,63]。内源性CYP450的耗竭会减弱间充质样TNBC细胞的转移表型[7]。相反,CYP2C19耗竭或任何化合物抑制剂治疗对基底样TNBC细胞的迁移和侵袭潜力没有显着影响,并且sEH的抑制未能诱导任何基底样TNBC细胞系中总EET水平的显着变化[7]。基底样TNBC的转移性和侵袭性能力与CYP450无关。CYP450介导的EET代谢与间充质样TNBC具有更强的相关性。
The expression or activity of targeted CYP450 has a more effective influence in reducing the metastatic burden of the mesenchymal-like TNBC subtype [7]. Meanwhile, HER2+ BC is found unaffected by either of the EETs. On the contrary, another study attributed the tamoxifen resistance in MDA-MB-361 (ER−/PR−/HER2+) to overexpression of CYP3A4, partly by enhancing 11,12-EET biosynthesis [64], while it is mentioned in the study by Maria Karmella Apaya that MCF-7 could not be affected by any EET [7]. Contradictory evidence reveals the existence of deeper complicated network mechanisms. Of note, sEH maintains a higher expression in hormone receptor-positive BC with the possible production of leukotoxins, further suggesting that sEH may be a key factor in the comorbidity of depression and hormone receptor-positive BC. sEH might promote the growth of BC by degrading epoxides and generating specific toxic diols, particularly in hormone receptor-positive BC.
4. Depression-Associated sEH Promotes Liver Dysfunction and Breast Cancer
As mentioned above, with the increase in depression exploration, extracerebral pathological alterations have been paid more and more attention. As the main organ of lipid metabolism, especially the metabolism of epoxide, the liver has been proposed to have an unexpected connection with brain functions [65,66,67,68,69]. The pathogenesis of Alzheimer’s disease (AD) is believed to be closely related to depression [70,71]. In particular, major depression and AD share biological processes and pathways, including 77 disease-related genes and 102 pathways [72]. Amyloid β (Aβ) is one of the vital shared pathogenic targets [73,74,75,76]. AD has been pointed out to be significantly associated with liver dysfunction [77]. The liver and kidney are the main extracerebral organs for the clearance of circulating Aβ [77]. Low-density lipoprotein receptor 1 (LRP-1), which is mediated through the liver, has been shown to regulate multiple tight junction proteins in the blood–brain barrier endothelium [78]. Studies also showed that those central mechanisms are involved in the neurological and endocrine systems of the brain leading to neuroendocrine regulation of CYP450 gene expression. Such mechanisms have been shown to involve the dopaminergic, noradrenergic, and 5-hydroxytryptaminergic systems of the brain with hypothalamic endocrine centers, among which repetitive restraint stress (RS) increases hepatic CYP2D1/2 activity in a stress-specific manner, while the main effectors of the stress system, glucocorticoids and epinephrine, are highly induced by CYP3A1/2 [79,80,81,82,83,84]. Epinephrine also induces the expression of CYP2C11 and CYP2D1/2. Studies have shown that human hepatocyte microsomes are the primary site of systemic CYP450, sEH-mediated epoxide metabolism [85]. This discovery further supports the importance of hepatocyte epoxide metabolism in depression–BC comorbidity.
Researchers also disclosed that psychiatric disorders induce liver dysfunction [77]. A study found that sEH in the livers of chronically stressed experimental animals increased specifically without its appearance in other organs and caused nonspecific changes in LOX and COX signaling pathways [86]. Furthermore, the specific knockdown of the hepatic sEH gene Ephx2 suppressed the expression of the depression-like phenotype. Moreover, the important hydrolase is predominantly expressed in hepatocytes [86]. The evidence suggests that hepatic sEH is one of the main causative factors of psychiatric disorders including depression. Additionally, sEH has been raised as the critical molecule in the brain–liver axis, with a positive correlation between sEH protein in the parietal cortex and sEH protein in the liver [87]. Thus, the downstream effects of hepatic epoxide metabolism, including inflammation, liver dysfunction, and lipid metabolisms, may be components of contributors to BC.
The effects of depression on the liver are known to be reflected in inflammation, oxidative damage, and reduced immune surveillance [65,69,88]. From the perspective of molecular mechanisms, the upregulation of hepatic sEH is one of the pivotal upstream causes of liver damage, liver fibrosis, and hepatitis [65,89,90,91]. The inhibition of hepatic sEH significantly reduces endoplasmic reticulum stress in hepatocytes and maintains low expression of prostaglandins and triglycerides, thereby reducing high-fat-diet-induced inflammation [92]. A notable observation is that overexpression of hepatic sEH increases liver triglyceride levels and hepatic inflammatory response [65,69].
Surprisingly, the induced expression of sEH in the liver only occurred in a long-term rather than short-term high-fat diet. Furthermore, sEH inhibition attenuates the high-fat-diet-induced plasma levels of proinflammatory cytokine increase and the adipocytic cytokine mRNA upregulation. It is thus clear that depression promotes specific upregulation of hepatic sEH, which is a potential inflammatory injury factor in the liver [92,93]. What is more, overexpression of sEH in the liver directly affects the balance of epoxide metabolism. The pathological changes in the liver are crucial to the disruption of the internal environmental homeostasis, which provides a potentially favorable environment for the development of BC.
Another noteworthy point is that ω-6 PUFAs and ω-3 PUFAs, as the precursors of DHA, EPA, AA, and LA, are clearly involved in the pathogenesis of depression [94]. Studies have shown that ω-3 PUFAs exert anti-inflammatory effects in the brain by regulating microglia function to maintain homeostasis, which improve fatty acids for the pathogen. At the same time, ω-6 PUFAs have been considered as promoting factors of inflammation, while the ability of dietary LA to increase the levels of inflammatory markers is influenced to some degree by the level of adiposity [94,95]. The ratio of ω-6 PUFAs/ω-3 PUFAs is considered to affect the balance of lipid metabolism [95,96]. A Mediterranean diet with high ω-3 PUFA levels has been mentioned to alleviate depressive symptoms and even decrease the prevalence of malignancies such as breast, lung, prostate, and colorectal cancers [97,98]. In addition, ω-3 PUFAs have been found to attenuate microglia-induced inflammation by inhibiting the HMGB1/TLR4/NF-κB pathway [99].
It has also been shown that an unhealthy diet can lead to obesity, which is highly correlated with a chronic inflammatory environment and depression. A high ratio of ω-6 PUFAs/ω-3 PUFAs has been demonstrated to be an unhealthy dietary pattern [100,101]. Western diets, another popular diet construction, show a high ω-6 PUFAs/ω-3 PUFAs ratio of 15/1 to 16.7/1 [100,102]. Western diets have been shown to induce hyperthrombotic and proinflammatory states [101,103,104,105,106]. ω-6 PUFAs and ω-3 PUFAs can be converted into EPA, DHA, AA, and LA after ingestion, which can be further converted into EETs, EpOMEs, EpETEs, and EpDPAs. This shift suggests that the Western diet promotes an imbalance in lipid metabolism, which leads to a range of proinflammatory and carcinogenic effects [22,23,107]. In addition, the dietary structure of high ω-6 PUFAs/ω-3 PUFAs is conducive to the growth and development of BC. Studies have discovered that LA is the most abundant polyunsaturated fatty acid in the Western diet [95,96], where the consumption of butter, corn oil, the rice plant (Oryza Sativa L.), and soybeans leads to significantly increased hepatic LA consumption and promotes LA metabolism via CYP450 (mainly by CYP2J2, CYP2C8, and CYP2C9) to produce 9, 10-EpOME (leukotoxin) and 12, 13-EpOME (isoleukotoxin) [108,109,110,111,112,113]. Another important fact is that stress is reported to induce the hepatic PXR expression, which is followed by induced hepatic CYP3A and CYP2C expression [81]. Therefore, the response of high dietary ω-6 PUFAs to depression results in high sEH expression promoting the production of 9, 10-DiHOME (leukotoxin diol) and 12, 13-DiHOME (isoleukotoxin diol) [55,82]. In addition, a high ω-6 PUFAs/ω-3 PUFAs diet may increase breast cancer risk [114,115,116,117]. Moreover, it has been shown that LA promotes the proliferation of MDA-MB-231 breast cancer cells by activating the EGFR/PI3K/Akt signaling pathway [118,119]. Meanwhile, AA has been found to have a similar effect as well [119]. It is worth noting that excessive LA intake has been implicated in the development of obesity and liver dysfunction [120,121,122,123,124,125,126]. This effect is fatal to BC patients, especially BC with obesity. Basically, hepatic sEH-mediated epoxide metabolism is an important mechanism by which the liver regulates the comorbidity of depression and BC.
Under the stimulation of chronic depression, chronic stress leads to the continuous increase in inflammatory cytokines leading to chronic persistent inflammation. Although the hypothalamic–adrenal axis is one of the important regulatory pathways of chronic inflammation induced by chronic stress, sEH-mediated epoxide metabolism is also an important regulatory pathway [83]. This is because EETs degraded by sEH are also important contributors to the creation of a proinflammatory endotrophic environment. Fatty liver has been identified as consistent with increased neuro-proinflammatory cytokines and amyloid β deposition in the brain of mice induced by a high-fat diet [127]. Thus, abnormal lipid metabolism and hepatic inflammation mediated by disturbances in hepatic epoxide metabolism are directly related to neuroinflammation and pathological alterations in amyloid β in the brain. Importantly, sEH upregulation increases EET degradation and promotes cytokine expression. A study illustrated that dual inhibitors of sEH and COX-2 improved hepatic fibrosis and portal hypertension and downregulated IL-6 levels, suggesting that IL-6 plays a driving role in hepatitis [128]. The prevalence of nonalcoholic fatty liver diseases (NAFLD) in patients with breast cancer is significantly higher than in healthy controls, while hepatic sEH is a key enzyme for NAFLD [129]. Moreover, breast cancer patients with NAFLD showed poorer prognosis in terms of recurrence [129,130].
In addition, as mentioned earlier, depression-mediated neuroinflammation is associated with IL-6, and there is also a positive relationship between this interleukin and the construction of TME in BC [131]. Therefore, IL-6-related pathways may be one of the downstream pathological developmental pathways of epoxide metabolic disorders. Currently, several studies are aimed at blocking the IL-6 receptor and its downstream signaling molecules to develop BC-related therapeutic regimens [29,132]. Generally, systematic chronic inflammation is a beneficial environment for BC development, which is tightly connected with adipose issues (especially cancer-associated adipocytes) [132,133,134,135,136]. This review points out that another potential target, blocking hepatic sEH rather than breast tissue sEH, might be a suppressor in partially comorbid BC and depression populations.
References
References
- Bray, F.; Msc, M.L.; Weiderpass, E.; Soerjomataram, I. The ever‐increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. https://doi.org/10.1002/cncr.33587.
- American Cancer Society. Cancer Facts & Figures 2021; American Cancer Society: Atlanta , 2021.
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. https://doi.org/10.3322/caac.21660.
- Huang, J.; Chan, P.S.; Lok, V.; Chen, X.; Ding, H.; Jin, Y.; Yuan, J.; Lao, X.-Q.; Zheng, Z.-J.; Wong, M.C. Global incidence and mortality of breast cancer: A trend analysis. Aging 2021, 13, 5748–5803. https://doi.org/10.18632/aging.202502.
- Barzaman, K.; Karami, J.; Zarei, Z.; Hosseinzadeh, A.; Kazemi, M.H.; Moradi-Kalbolandi, S.; Safari, E.; Farahmand, L. Breast cancer: Biology, biomarkers, and treatments. Immunopharmacol. 2020, 84, 106535. https://doi.org/10.1016/j.intimp.2020.106535.
- Liedtke, C.; Mazouni, C.; Hess, K.R.; André, F.; Tordai, A.; Mejia, J.A.; Symmans, W.F.; Gonzalez-Angulo, A.M.; Hennessy, B.; Green, M.; et al. Response to Neoadjuvant Therapy and Long-Term Survival in Patients With Triple-Negative Breast Cancer. Clin. Oncol. 2008, 26, 1275–1281. https://doi.org/10.1200/jco.2007.14.4147.
- Apaya, M.K.; Shiau, J.-Y.; Liao, G.-S.; Liang, Y.-J.; Chen, C.-W.; Yang, H.-C.; Chu, C.-H.; Yu, J.-C.; Shyur, L.-F. Integrated omics-based pathway analyses uncover CYP epoxygenase-associated networks as theranostic targets for metastatic triple negative breast cancer. Exp. Clin. Cancer Res. 2019, 38, 1–22. https://doi.org/10.1186/s13046-019-1187-y.
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. Clin. Investig. 2011, 121, 2750–2767. https://doi.org/10.1172/jci45014.
- Puigpinós-Riera, R.; Graells-Sans, A.; Serral, G.; Continente, X.; Bargalló, X.; Domènech, M.; Espinosa-Bravo, M.; Grau, J.; Macià, F.; Manzanera, R.; et al. Anxiety and depression in women with breast cancer: Social and clinical determinants and influence of the social network and social support (DAMA cohort). Cancer Epidemiology 2018, 55, 123–129. https://doi.org/10.1016/j.canep.2018.06.002.
- Kissane, D.W.; Grabsch, B.; Love, A.; Clarke, D.M.; Bloch, S.; Smith, G.C. Psychiatric Disorder in Women with Early Stage and Advanced Breast Cancer: A Comparative Analysis. New Zealand J. Psychiatry 2004, 38, 320–326. https://doi.org/10.1080/j.1440-1614.2004.01358.x.
- Pelletier, G.; Verhoef, M.J.; Khatri, N.; Hagen, N. Quality of Life in Brain Tumor Patients: The Relative Contributions of Depression, Fatigue, Emotional Distress, and Existential Issues. Neuro-Oncology 2002, 57, 41–49. https://doi.org/10.1023/a:1015728825642.
- Desai, M.; Bruce, M.L.; Kasl, S.V. The Effects of Major Depression and Phobia on Stage at Diagnosis of Breast Cancer. J. Psychiatry Med. 1999, 29, 29–45. https://doi.org/10.2190/0c63-u15v-5nur-tvxe.
- Satin, J.R.; Linden, W.; Phillips, M.J. Depression as a predictor of disease progression and mortality in cancer patients. Cancer 2009, 115, 5349–5361. https://doi.org/10.1002/cncr.24561.
- Inagaki, M.; Yoshikawa, E.; Matsuoka, Y.; Sugawara, Y.; Nakano, T.; Akechi, T.; Wada, N.; Imoto, S.; Murakami, K.; Uchitomi, Y.; et al. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer 2006, 109, 146–156. https://doi.org/10.1002/cncr.22368.
- Dietrich, J.; Han, R.; Yang, Y.; Mayer-Pröschel, M.; Noble, M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. Biol. 2006, 5, 22–22. https://doi.org/10.1186/jbiol50.
- Janelsins, M.C.; Roscoe, J.A.; Berg, M.J.; Thompson, B.D.; Gallagher, M.J.; Morrow, G.R.; Heckler, C.E.; Jean-Pierre, P.; Opanashuk, L.A.; Gross, R.A. IGF-1 Partially Restores Chemotherapy-Induced Reductions in Neural Cell Proliferation in Adult C57BL/6 Mice. Cancer Investig. 2009, 28, 544–553. https://doi.org/10.3109/07357900903405942.
- Christie, L.-A.; Acharya, M.M.; Parihar, V.K.; Nguyen, A.; Martirosian, V.; Limoli, C.L. Impaired Cognitive Function and Hippocampal Neurogenesis following Cancer Chemotherapy. Cancer Res. 2012, 18, 1954–1965. https://doi.org/10.1158/1078-0432.ccr-11-2000.
- Chapman, B.; Swainston, J.; Grunfeld, E.A.; Derakshan, N. COVID-19 Outbreak Effects on Job Security and Emotional Functioning Amongst Women Living With Breast Cancer. Psychol. 2020, 11, 582014. https://doi.org/10.3389/fpsyg.2020.582014.
- Xu, C.; Ganesan, K.; Liu, X.; Ye, Q.; Cheung, Y.; Liu, D.; Zhong, S.; Chen, J. Prognostic Value of Negative Emotions on the Incidence of Breast Cancer: A Systematic Review and Meta-Analysis of 129,621 Patients with Breast Cancer. Cancers 2022, 14, 475. https://doi.org/10.3390/cancers14030475.
- Bortolato, B.; Hyphantis, T.N.; Valpione, S.; Perini, G.; Maes, M.; Morris, G.; Kubera, M.; Köhler, C.A.; Fernandes, B.S.; Stubbs, B.; et al. Depression in cancer: The many biobehavioral pathways driving tumor progression. Cancer Treat. Rev. 2016, 52, 58–70. https://doi.org/10.1016/j.ctrv.2016.11.004.
- Perez-Tejada, J.; Labaka, A.; Vegas, O.; Larraioz, A.; Pescador, A.; Arregi, A. Anxiety and depression after breast cancer: The predictive role of monoamine levels. J. Oncol. Nurs. 2021, 52, 101953. https://doi.org/10.1016/j.ejon.2021.101953.
- Newman, J.W.; Morisseau, C.; Hammock, B.D. Epoxide hydrolases: Their roles and interactions with lipid metabolism. Lipid Res. 2005, 44, 1–51. https://doi.org/10.1016/j.plipres.2004.10.001.
- Morisseau, C. Role of epoxide hydrolases in lipid metabolism. Biochime 2013, 95, 91–95. https://doi.org/10.1016/j.biochi.2012.06.011.
- Hashimoto, K. Role of Soluble Epoxide Hydrolase in Metabolism of PUFAs in Psychiatric and Neurological Disorders. Pharmacol. 2019, 10, 36. https://doi.org/10.3389/fphar.2019.00036.
- Markaverich, B.M.; Crowley, J.R.; Alejandro, M.A.; Shoulars, K.; Casajuna, N.; Mani, S.; Reyna, A.; Sharp, J. Leukotoxin Diols from Ground Corncob Bedding Disrupt Estrous Cyclicity in Rats and Stimulate MCF-7 Breast Cancer Cell Proliferation. Heal. Perspect. 2005, 113, 1698–1704. https://doi.org/10.1289/ehp.8231.
- Markaverich, B.; Mani, S.; Alejandro, M.A.; Mitchell, A.; Markaverich, D.; Brown, T.; Velez-Trippe, C.; Murchison, C.; O'Malley, B.; Faith, R. A novel endocrine-disrupting agent in corn with mitogenic activity in human breast and prostatic cancer cells.. Heal. Perspect. 2002, 110, 169–177. https://doi.org/10.1289/ehp.02110169.
- Fagundes, C.P.; Glaser, R.; Hwang, B.S.; Malarkey, W.B.; Kiecolt-Glaser, J.K. Depressive symptoms enhance stress-induced inflammatory responses. Brain, Behav. Immun. 2012, 31, 172–176. https://doi.org/10.1016/j.bbi.2012.05.006.
- Ibrahim, S.A.; Gadalla, R.; El-Ghonaimy, E.A.; Samir, O.; Mohamed, H.T.; Hassan, H.; Greve, B.; El-Shinawi, M.; Mohamed, M.M.; Götte, M. Syndecan-1 is a novel molecular marker for triple negative inflammatory breast cancer and modulates the cancer stem cell phenotype via the IL-6/STAT3, Notch and EGFR signaling pathways. Cancer 2017, 16, 1–19. https://doi.org/10.1186/s12943-017-0621-z.
- Weng, Y.-S.; Tseng, H.-Y.; Chen, Y.-A.; Shen, P.-C.; Al Haq, A.T.; Chen, L.-M.; Tung, Y.-C.; Hsu, H.-L. MCT-1/miR-34a/IL-6/IL-6R signaling axis promotes EMT progression, cancer stemness and M2 macrophage polarization in triple-negative breast cancer. Cancer 2019, 18, 1–15. https://doi.org/10.1186/s12943-019-0988-0.
- Wang, F.; Liu, J.; Liu, L.; Wang, F.; Ma, Z.; Gao, D.; Zhang, Q.; Yu, Z. The status and correlates of depression and anxiety among breast-cancer survivors in Eastern China: A population-based, cross-sectional case–control study. BMC Public Heal. 2014, 14, 326–326. https://doi.org/10.1186/1471-2458-14-326.
- Eng, J.W.-L.; Kokolus, K.M.; Reed, C.B.; Hylander, B.L.; Ma, W.W.; Repasky, E.A. A nervous tumor microenvironment: The impact of adrenergic stress on cancer cells, immunosuppression, and immunotherapeutic response. Cancer Immunol. Immunother. 2014, 63, 1115–1128. https://doi.org/10.1007/s00262-014-1617-9.
- Gosain, R.; Gage-Bouchard, E.; Ambrosone, C.; Repasky, E.; Gandhi, S. Stress reduction strategies in breast cancer: Review of pharmacologic and non-pharmacologic based strategies. Immunopathol. 2020, 42, 719–734. https://doi.org/10.1007/s00281-020-00815-y.
- Kach, J.; Conzen, S.D.; Szmulewitz, R.Z. Targeting the glucocorticoid receptor in breast and prostate cancers. Transl. Med. 2015, 7, 305ps19. https://doi.org/10.1126/scitranslmed.aac7531.
- Van Der Pompe, G.; Antoni, M.H.; Mulder, C.L.; Heijnen, C.; Goodkin, K.; De Graeff, A.; Garssen, B.; De Vries, M.J. Psychoneuroimmunology and the course of breast cancer: An overview the impact of psychosocial factors on progression of breast cancer through immune and endocrine mechanisms. Psycho-Oncology 1994, 3, 271–288. https://doi.org/10.1002/pon.2960030404.
- Prinz, M.; Priller, J. Microglia and brain macrophages in the molecular age: From origin to neuropsychiatric disease. Rev. Neurosci. 2014, 15, 300–312. https://doi.org/10.1038/nrn3722.
- Pace, T.W.; Mletzko, T.C.; Alagbe, O.; Musselman, D.L.; Nemeroff, C.B.; Miller, A.H.; Heim, C.M. Increased Stress-Induced Inflammatory Responses in Male Patients With Major Depression and Increased Early Life Stress. J. Psychiatry 2006, 163, 1630–1633. https://doi.org/10.1176/ajp.2006.163.9.1630.
- Arendt, L.M.; McCready, J.; Keller, P.J.; Baker, D.D.; Naber, S.P.; Seewaldt, V.; Kuperwasser, C. Obesity Promotes Breast Cancer by CCL2-Mediated Macrophage Recruitment and Angiogenesis. Cancer Res. 2013, 73, 6080–6093. https://doi.org/10.1158/0008-5472.can-13-0926.
- Santander, A.M.; Lopez-Ocejo, O.; Casas, O.; Agostini, T.; Sanchez, L.; Lamas-Basulto, E.; Carrio, R.; Cleary, M.P.; Gonzalez-Perez, R.R.; Torroella-Kouri, M. Paracrine Interactions between Adipocytes and Tumor Cells Recruit and Modify Macrophages to the Mammary Tumor Microenvironment: The Role of Obesity and Inflammation in Breast Adipose Tissue. Cancers 2015, 7, 143–178. https://doi.org/10.3390/cancers7010143.
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Immunol. 2010, 11, 889–896. https://doi.org/10.1038/ni.1937.
- Dirkx, A.E.M.; Egbrink, M.G.A.O.; Wagstaff, J.; Griffioen, A.W. Monocyte/macrophage infiltration in tumors: Modulators of angiogenesis. Leukoc. Biol. 2006, 80, 1183–1196. https://doi.org/10.1189/jlb.0905495.
- Junjeong, C.; Jones, G.; Haerin, J.; Seung, K.J. The role of tumor-associated macrophage in breast cancer biology. Histopathol. 2017, 33, 133–145. https://doi.org/10.14670/HH-11-916.
- Lewis, C.E.; Pollard, J.W. Distinct Role of Macrophages in Different Tumor Microenvironments. Cancer Res. 2006, 66, 605–612. https://doi.org/10.1158/0008-5472.can-05-4005.
- Alhallak, I.; Wolter, K.G.; Munoz, A.C.; A Simmen, F.; Ward, R.J.; A Petty, S.; Li, L.-X.; Simmen, R.C. Breast adipose regulation of premenopausal breast epithelial phenotype involves interleukin 10. Mol. Endocrinol. 2021, 67, 173–188. https://doi.org/10.1530/jme-21-0100.
- Qiu, X.; Zhao, T.; Luo, R.; Qiu, R.; Li, Z. Tumor-Associated Macrophages: Key Players in Triple-Negative Breast Cancer. Oncol. 2022, 12, 772615. https://doi.org/10.3389/fonc.2022.772615.
- Lucas, C.; Workman, C.J.; Beyaz, S.; LoCascio, S.; Zhao, G.; Vignali, D.A.A.; Sykes, M. LAG-3, TGF-β, and cell-intrinsic PD-1 inhibitory pathways contribute to CD8 but not CD4 T-cell tolerance induced by allogeneic BMT with anti-CD40L. Blood 2011, 117, 5532–5540. https://doi.org/10.1182/blood-2010-11-318675.
- Chu, P.-Y.; Wang, S.-M.; Chen, P.-M.; Tang, F.-Y.; Chiang, E.-P.I. Expression of MTDH and IL-10 Is an Independent Predictor of Worse Prognosis in ER-Negative or PR-Negative Breast Cancer Patients. Clin. Med. 2020, 9, 3153. https://doi.org/10.3390/jcm9103153.
- Chen, K.-Y.; Chien, W.-C.; Liao, J.-M.; Tsai, C.-W.; Chang, W.-S.; Su, C.-H.; Hsu, S.-W.; Wang, H.-C.; Bau, D.-T. Contribution of Interleukin-10 Genotype to Triple Negative Breast Cancer Risk. Anticancer Res. 2021, 41, 2451–2457. https://doi.org/10.21873/anticanres.15020.
- Kiecolt-Glaser, J.K.; Derry, H.M.; Fagundes, C.P. Inflammation: Depression Fans the Flames and Feasts on the Heat. J. Psychiatry 2015, 172, 1075–1091. https://doi.org/10.1176/appi.ajp.2015.15020152.
- Lamers, F.; Milaneschi, Y.; Smit, J.H.; Schoevers, R.A.; Wittenberg, G.; Penninx, B.W. Longitudinal Association Between Depression and Inflammatory Markers: Results From the Netherlands Study of Depression and Anxiety. Psychiatry 2019, 85, 829–837. https://doi.org/10.1016/j.biopsych.2018.12.020.
- Ng, A.; Tam, W.W.; Zhang, M.W.; Ho, C.S.; Husain, S.F.; McIntyre, R.S.; Ho, R. IL-1β, IL-6, TNF- α and CRP in Elderly Patients with Depression or Alzheimer’s disease: Systematic Review and Meta-Analysis. Rep. 2018, 8, 1–12. https://doi.org/10.1038/s41598-018-30487-6.
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A Meta-Analysis of Cytokines in Major Depression. Psychiatry 2010, 67, 446–457. https://doi.org/10.1016/j.biopsych.2009.09.033.
- Uzzan, S.; Azab, A. Anti-TNF-α Compounds as a Treatment for Depression. Molecules 2021, 26, 2368. https://doi.org/10.3390/molecules26082368.
- Cruceriu, D.; Baldasici, O.; Balacescu, O.; Berindan-Neagoe, I. The dual role of tumor necrosis factor-alpha (TNF-α) in breast cancer: Molecular insights and therapeutic approaches. Oncol. 2020, 43, 1–18. https://doi.org/10.1007/s13402-019-00489-1.
- Panigrahy, D.; Kaipainen, A.; Greene, E.R.; Huang, S. Cytochrome P450-derived eicosanoids: The neglected pathway in cancer. Cancer Metastasis Rev. 2010, 29, 723–735. https://doi.org/10.1007/s10555-010-9264-x.
- Panigrahy, D.; Edin, M.L.; Lee, C.; Huang, S.; Bielenberg, D.R.; Butterfield, C.E.; Barnés, C.M.; Mammoto, A.; Mammoto, T.; Luria, A.; et al. Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice. Clin. Investig. 2012, 122, 178–191. https://doi.org/10.1172/jci58128.
- Luo, J.; Yao, J.F.; Deng, X.F.; Zheng, X.D.; Jia, M.; Wang, Y.Q.; Huang, Y.; Zhu, J.H. 14, 15-EET induces breast cancer cell EMT and cisplatin resistance by up-regulating integrin αvβ3 and activating FAK/PI3K/AKT signaling. Exp. Clin. Cancer Res. 2018, 37, 1–11. https://doi.org/10.1186/s13046-018-0694-6.
- Zhu, P.; Peck, B.; Licea-Perez, H.; Callahan, J.F.; Booth-Genthe, C. Development of a semi-automated LC/MS/MS method for the simultaneous quantitation of 14,15-epoxyeicosatrienoic acid, 14,15-dihydroxyeicosatrienoic acid, leukotoxin and leukotoxin diol in human plasma as biomarkers of soluble epoxide hydrolase activity in vivo. Chromatogr. B 2011, 879, 2487–2493. https://doi.org/10.1016/j.jchromb.2011.06.042.
- Hildreth, K.; Kodani, S.D.; Hammock, B.D.; Zhao, L. Cytochrome P450-derived linoleic acid metabolites EpOMEs and DiHOMEs: A review of recent studies. Nutr. Biochem. 2020, 86, 108484. https://doi.org/10.1016/j.jnutbio.2020.108484.
- Moghaddam, M.F.; Grant, D.F.; Cheek, J.M.; Greene, J.F.; Williamson, K.C.; Hammock, B.D. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Med. 1997, 3, 562–566. https://doi.org/10.1038/nm0597-562.
- Rody, A.; Karn, T.; Liedtke, C.; Pusztai, L.; Ruckhaeberle, E.; Hanker, L.; Gaetje, R.; Solbach, C.; Ahr, A.; Metzler, D.; et al. A clinically relevant gene signature in triple negative and basal-like breast cancer. Breast Cancer Res. 2011, 13, R97–R97. https://doi.org/10.1186/bcr3035.
- Sørlie, T.; Wang, Y.; Xiao, C.; Johnsen, H.; Naume, B.; Samaha, R.R.; Børresen-Dale, A.-L. Distinct molecular mechanisms underlying clinically relevant subtypes of breast cancer: Gene expression analyses across three different platforms. BMC Genom. 2006, 7, 127–127. https://doi.org/10.1186/1471-2164-7-127.
- Paquet, E.; Hallett, M.T. Absolute Assignment of Breast Cancer Intrinsic Molecular Subtype. JNCI: J. Natl. Cancer Inst. 2014, 107, 357. https://doi.org/10.1093/jnci/dju357.
- Badve, S.; Dabbs, D.J.; Schnitt, S.J.; Baehner, F.L.; Decker, T.; Eusebi, V.; Fox, S.B.; Ichihara, S.; Jacquemier, J.; Lakhani, S.R.; et al. Basal-like and triple-negative breast cancers: A critical review with an emphasis on the implications for pathologists and oncologists. Pathol. 2010, 24, 157–167. https://doi.org/10.1038/modpathol.2010.200.
- Phuong, N.T.T.; Kim, J.W.; Kim, J.-A.; Jeon, J.S.; Lee, J.-Y.; Xu, W.J.; Yang, J.W.; Kim, S.K.; Kang, K.W. Role of the CYP3A4-mediated 11,12-epoxyeicosatrienoic acid pathway in the development of tamoxifen-resistant breast cancer. Oncotarget 2017, 8, 71054–71069. https://doi.org/10.18632/oncotarget.20329.
- Lee, J.W.; Park, S.H. Association between depression and nonalcoholic fatty liver disease: Contributions of insulin resistance and inflammation. Affect. Disord. 2020, 278, 259–263. https://doi.org/10.1016/j.jad.2020.09.073.
- Bhardwaj, P.; Brown, K.A. Obese Adipose Tissue as a Driver of Breast Cancer Growth and Development: Update and Emerging Evidence. Oncol. 2021, 11, 638918. https://doi.org/10.3389/fonc.2021.638918.
- Ng, C.H.; Lim, W.H.; Lim, X.C.; Xiao, J.; Tan, D.J.H.; Syn, N.; Ho, C.S.H.; Kow, A.W.C.; Tan, E.X.X.; Fung, J.; et al. A meta‐analysis on the incidence of donor‐related depression after liver transplant. Int. 2021, 34, 2061–2070. https://doi.org/10.1111/tri.13975.
- Cho, I.Y.; Chang, Y.; Sung, E.; Kang, J.-H.; Wild, S.H.; Byrne, C.D.; Shin, H.; Ryu, S. Depression and increased risk of non-alcoholic fatty liver disease in individuals with obesity. Epidemiology Psychiatr. Sci. 2021, 30, e23. https://doi.org/10.1017/s204579602000116x.
- Orrù, M.G.; Pariante, C.M. Depression and liver diseases. Liver Dis. 2005, 37, 564–565. https://doi.org/10.1016/j.dld.2005.04.003.
- Sierksma, A.S.; Hove, D.L.V.D.; Steinbusch, H.W.; Prickaerts, J. Major depression, cognitive dysfunction and Alzheimer's disease: Is there a link?. J. Pharmacol. 2010, 626, 72–82. https://doi.org/10.1016/j.ejphar.2009.10.021.
- Caraci, F.; Copani, A.; Nicoletti, F.; Drago, F. Depression and Alzheimer's disease: Neurobiological links and common pharmacological targets. J. Pharmacol. 2009, 626, 64–71. https://doi.org/10.1016/j.ejphar.2009.10.022.
- Guo, P.; Chen, S.; Wang, H.; Wang, Y.; Wang, J. A Systematic Analysis on the Genes and Their Interaction Underlying the Comorbidity of Alzheimer's Disease and Major Depressive Disorder. Aging Neurosci. 2022, 13, 789698 https://doi.org/10.3389/fnagi.2021.789698.
- Baba, H.; Nakano, Y.; Maeshima, H.; Satomura, E.; Kita, Y.; Suzuki, T.; Arai, H. Metabolism of Amyloid-β Protein May Be Affected in Depression. Clin. Psychiatry 2011, 73, 115–120. https://doi.org/10.4088/jcp.10m06766.
- Kita, Y.; Baba, H.; Maeshima, H.; Nakano, Y.; Suzuki, T.; Arai, H. Serum amyloid β protein in young and elderly depression: A pilot study. Psychogeriatrics 2009, 9, 180–185. https://doi.org/10.1111/j.1479-8301.2009.00293.x.
- Pandolfo, G.; Iannuzzo, F.; Genovese, G.; Bruno, A.; Pioggia, G.; Baldari, S.; Gangemi, S. Mental Illness and Amyloid: A Scoping Review of Scientific Evidence over the Last 10 Years (2011 to 2021). Brain Sci. 2021, 11, 1352. https://doi.org/10.3390/brainsci11101352.
- Qiu, W.Q.; Zhu, H.; Dean, M.; Liu, Z.; Vu, L.; Fan, G.; Li, H.; Mwamburi, M.; Steffens, D.C.; Au, R. Amyloid-associated depression and ApoE4 allele: Longitudinal follow-up for the development of Alzheimer's disease. J. Geriatr. Psychiatry 2015, 31, 316–322. https://doi.org/10.1002/gps.4339.
- Estrada, L.D.; Ahumada, P.; Cabrera, D.; Arab, J.P. Liver Dysfunction as a Novel Player in Alzheimer’s Progression: Looking Outside the Brain. Aging Neurosci. 2019, 11, 174. https://doi.org/10.3389/fnagi.2019.00174.
- Zhao, Y.; Li, D.; Zhao, J.; Song, J.; Zhao, Y. The role of the low-density lipoprotein receptor–related protein 1 (LRP-1) in regulating blood-brain barrier integrity. Neurosci. 2016, 27, 623–634. https://doi.org/10.1515/revneuro-2015-0069.
- Daskalopoulos, E.P.; Malliou, F.; Rentesi, G.; Marselos, M.; Lang, M.A.; Konstandi, M. Stress is a critical player inCYP3A,CYP2C, andCYP2Dregulation: Role of adrenergic receptor signaling pathways. J. Physiol. Metab. 2012, 303, E40–E54. https://doi.org/10.1152/ajpendo.00545.2011.
- Bromek, E.; Daniel, W.A. The regulation of liver cytochrome P450 expression and activity by the brain serotonergic system in different experimental models. Expert Opin. Drug Metab. Toxicol. 2021, 17, 413–424. https://doi.org/10.1080/17425255.2021.1872543.
- Bromek, E.; Wójcikowski, J.; Daniel, W.A. Involvement of the paraventricular (PVN) and arcuate (ARC) nuclei of the hypothalamus in the central noradrenergic regulation of liver cytochrome P450. Pharmacol. 2013, 86, 1614–1620. https://doi.org/10.1016/j.bcp.2013.09.006.
- Sadakierska-Chudy, A.; Haduch, A.; Rysz, M.; Gołembiowska, K.; Daniel, W.A. The role of brain noradrenergic system in the regulation of liver cytochrome P450 expression. Pharmacol. 2013, 86, 800–807. https://doi.org/10.1016/j.bcp.2013.07.017.
- Wójcikowski, J.; Daniel, W.A. The brain dopaminergic system as an important center regulating liver cytochrome P450 in the rat. Expert Opin. Drug Metab. Toxicol. 2009, 5, 631–645. https://doi.org/10.1517/17425250902973703.
- Danek, P.; Kuban, W.; Daniel, W. The Effect of Chronic Iloperidone Treatment on Cytochrome P450 Expression and Activity in the Rat Liver: Involvement of Neuroendocrine Mechanisms. J. Mol. Sci. 2021, 22, 8447. https://doi.org/10.3390/ijms22168447.
- Rifkind, A.B.; Lee, C.; Chang, T.K.; Waxman, D.J. Arachidonic acid metabolism by human cytochrome P450s 2C8, 2C9, 2E1, and 1A2: Regioselective oxygenation and evidence for a role for CYP2C enzymes in arachidonic acid epoxygenation in human liver microsomes. Biochem. Biophys. 1995, 320, 380–389. https://doi.org/10.1016/0003-9861(95)90023-3.
- Qin, X.-H.; Wu, Z.; Dong, J.-H.; Zeng, Y.-N.; Xiong, W.-C.; Liu, C.; Wang, M.-Y.; Zhu, M.-Z.; Chen, W.-J.; Zhang, Y.; et al. Liver Soluble Epoxide Hydrolase Regulates Behavioral and Cellular Effects of Chronic Stress. Cell Rep. 2019, 29, 3223–3234.e6. https://doi.org/10.1016/j.celrep.2019.11.006.
- Zhang, J.; Tan, Y.; Chang, L.; Hammock, B.D.; Hashimoto, K. Increased expression of soluble epoxide hydrolase in the brain and liver from patients with major psychiatric disorders: A role of brain—Liver axis. Affect. Disord. 2020, 270, 131–134. https://doi.org/10.1016/j.jad.2020.03.070.
- Jung, J.Y.; Park, S.K.; Oh, C.-M.; Chung, P.-W.; Ryoo, J.-H. Non-Alcoholic Fatty Liver Disease and Its Association with Depression in Korean General Population. Korean Med. Sci. 2019, 34, e199. https://doi.org/10.3346/jkms.2019.34.e199.
- Harris, T.R.; Bettaieb, A.; Kodani, S.; Dong, H.; Myers, R.; Chiamvimonvat, N.; Haj, F.G.; Hammock, B.D. Inhibition of soluble epoxide hydrolase attenuates hepatic fibrosis and endoplasmic reticulum stress induced by carbon tetrachloride in mice. Appl. Pharmacol. 2015, 286, 102–111. https://doi.org/10.1016/j.taap.2015.03.022.
- Yao, L.; Cao, B.; Cheng, Q.; Cai, W.; Ye, C.; Liang, J.; Liu, W.; Tan, L.; Yan, M.; Li, B.; et al. Inhibition of soluble epoxide hydrolase ameliorates hyperhomocysteinemia-induced hepatic steatosis by enhancing β-oxidation of fatty acid in mice. J. Physiol. Liver Physiol. 2019, 316, G527–G538. https://doi.org/10.1152/ajpgi.00148.2018.
- Mello, A.; Hsu, M.-F.; Koike, S.; Chu, B.; Cheng, J.; Yang, J.; Morisseau, C.; Torok, N.J.; Hammock, B.D.; Haj, F.G. Soluble Epoxide Hydrolase Hepatic Deficiency Ameliorates Alcohol-Associated Liver Disease. Mol. Gastroenterol. Hepatol. 2021, 11, 815–830. https://doi.org/10.1016/j.jcmgh.2020.10.002.
- Liu, Y.; Dang, H.; Li, D.; Pang, W.; Hammock, B.D.; Zhu, Y. Inhibition of Soluble Epoxide Hydrolase Attenuates High-Fat-Diet–Induced Hepatic Steatosis by Reduced Systemic Inflammatory Status in Mice. PLoS ONE 2012, 7, e39165. https://doi.org/10.1371/journal.pone.0039165.
- Warner, J.; Hardesty, J.; Zirnheld, K.; McClain, C.; Warner, D.; Kirpich, I. Soluble Epoxide Hydrolase Inhibition in Liver Diseases: A Review of Current Research and Knowledge Gaps. Biology 2020, 9, 124. https://doi.org/10.3390/biology9060124.
- Rodríguez-Iglesias, N.; Nadjar, A.; Sierra, A.; Valero, J. Susceptibility of Female Mice to the Dietary Omega-3/Omega-6 Fatty-Acid Ratio: Effects on Adult Hippocampal Neurogenesis and Glia. J. Mol. Sci. 2022, 23, 3399. https://doi.org/10.3390/ijms23063399.
- Simopoulos, A. Importance of the Ratio of Omega-6/Omega-3 Essential Fatty Acids: Evolutionary Aspects. World Rev. Nutr. Diet. 2003, 92, 1–22. https://doi.org/10.1159/000073788.
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Pharmacother. 2002, 56, 365–379. https://doi.org/10.1016/s0753-3322(02)00253-6.
- Fabian, C.J.; Kimler, B.F.; Hursting, S.D. Omega-3 fatty acids for breast cancer prevention and survivorship. Breast Cancer Res. 2015, 17, 1–11. https://doi.org/10.1186/s13058-015-0571-6.
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Rev. Food Sci. Technol. 2018, 9, 345–381. https://doi.org/10.1146/annurev-food-111317-095850.
- Chen, X.; Wu, S.; Chen, C.; Xie, B.; Fang, Z.; Hu, W.; Chen, J.; Fu, H.; He, H. Omega-3 polyunsaturated fatty acid supplementation attenuates microglial-induced inflammation by inhibiting the HMGB1/TLR4/NF-κB pathway following experimental traumatic brain injury. Neuroinflammation 2017, 14, 1–12. https://doi.org/10.1186/s12974-017-0917-3.
- Simopoulos, A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: Nutritional implications for chronic diseases. Pharmacother. 2006, 60, 502–507. https://doi.org/10.1016/j.biopha.2006.07.080.
- Sellem, F.; Pesando, D.; Bodennec, G.; Girard, J.-P.; Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. https://doi.org/10.3390/nu8030128.
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.; Stanton, C. Health Implications of High Dietary Omega-6 Polyunsaturated Fatty Acids. Nutr. Metab. 2012, 2012, 1–16. https://doi.org/10.1155/2012/539426.
- Simopoulos, A.P. The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases. Biol. Med. 2008, 233, 674–688. https://doi.org/10.3181/0711-mr-311.
- Kang, J.X. The importance of omega-6/omega-3 fatty acid ratio in cell function. The gene transfer of omega-3 fatty acid desaturase. World Rev. Nutr. Dietetics. 2003, 92, 23–36.
- Simopoulos, A.P. Evolutionary Aspects of Diet: Essential Fatty Acids. World Rev. Nutr. https://doi.org/10.1385/1-59259-119-1:3.
- Ramos-Lopez, O.; Martinez-Urbistondo, D.; Vargas-Nuñez, J.A.; Martinez, J.A. The Role of Nutrition on Meta-inflammation: Insights and Potential Targets in Communicable and Chronic Disease Management. Obes. Rep. 2022, 22, 1–31. https://doi.org/10.1007/s13679-022-00490-0.
- Trovato, F.M.; Martines, G.F.; Catalano, D. Addressing Western dietary pattern in obesity and NAFLD. Nutrire 2018, 43, 11. https://doi.org/10.1186/s41110-018-0067-0.
- Yang, J.; Solaimani, P.; Dong, H.; Hammock, B.; Hankinson, O. Treatment of mice with 2,3,7,8-Tetrachlorodibenzo-p-dioxin markedly increases the levels of a number of cytochrome P450 metabolites of omega-3 polyunsaturated fatty acids in the liver and lung. Toxicol. Sci. 2013, 38, 833–836. https://doi.org/10.2131/jts.38.833.
- Fan, R.; Kim, J.; You, M.; Giraud, D.; Toney, A.M.; Shin, S.-H.; Kim, S.-Y.; Borkowski, K.; Newman, J.W.; Chung, S. α-Linolenic acid-enriched butter attenuated high fat diet-induced insulin resistance and inflammation by promoting bioconversion of n-3 PUFA and subsequent oxylipin formation. Nutr. Biochem. 2019, 76, 108285–108285. https://doi.org/10.1016/j.jnutbio.2019.108285.
- Ansari, M.H.; Ahmad, S.; Ahmad, F.; Ahmad, M.; Osman, S.M. Co‐occurrence of Coronaric and Vernolic Acids in Compositae Seed Oils. 1987, 89, 116–118. https://doi.org/10.1002/lipi.19870890307.
- Powell, R.G.; Smith, C.R.; Wolff, I.A. cis-5,cis-9,cis-12-octadecatrienoic and some unusual oxygenated acids inXeranthemum annuum seed oil. Lipids 1967, 2, 172–177. https://doi.org/10.1007/bf02530918.
- Kato, T.; Yamaguchi, Y.; Uyehara, T.; Yokoyama, T.; Namai, T.; Yamanaka, S. Self defensive substances in rice plant against rice blast disease. Tetrahedron Lett. 1983, 24, 4715–4718. https://doi.org/10.1016/s0040-4039(00)86236-x.
- Deol, S.; Fahrmann, J.; Yang, J.; Evans, J.R.; Rizo, A.; Grapov, D.; Salemi, M.; Wanichthanarak, K.; Fiehn, O.; Phinney, B.; et al. Omega-6 and omega-3 oxylipins are implicated in soybean oil-induced obesity in mice. Rep. 2017, 7, 1–13. https://doi.org/10.1038/s41598-017-12624-9.
- Burns, J.L.; Nakamura, M.T.; Ma, D.W.L. Differentiating the biological effects of linoleic acid from arachidonic acid in health and disease. Prostaglandins, Leukot. Essent. Fat. Acids 2018, 135, 1–4. https://doi.org/10.1016/j.plefa.2018.05.004.
- Bassett, J.K.; Hodge, A.M.; English, D.R.; MacInnis, R.J.; Giles, G.G. Plasma phospholipids fatty acids, dietary fatty acids, and breast cancer risk. Cancer Causes Control 2016, 27, 759–773. https://doi.org/10.1007/s10552-016-0753-2.
- Goodstine, S.L.; Zheng, T.; Holford, T.R.; Ward, B.A.; Carter, D.; Owens, P.H.; Mayne, S.T. Dietary (n-3)/(n-6) fatty acid ratio: Possible relationship to premenopausal but not postmenopausal breast cancer risk in U.S. women.. Nutr. 2003, 133, 1409–1414. https://doi.org/10.1093/jn/133.5.1409.
- Murff, H.J.; Shu, X.-O.; Li, H.; Yang, G.; Wu, X.; Cai, H.; Wen, W.; Gao, Y.-T.; Zheng, W. Dietary polyunsaturated fatty acids and breast cancer risk in Chinese women: A prospective cohort study. J. Cancer 2010, 128, 1434–1441. https://doi.org/10.1002/ijc.25703.
- Serna-Marquez, N.; Diaz-Aragon, R.; Reyes-Uribe, E.; Cortes-Reynosa, P.; Salazar, E.P. Linoleic acid induces migration and invasion through FFAR4- and PI3K-/Akt-dependent pathway in MDA-MB-231 breast cancer cells. Med Oncol. 2017, 34, 1–12. https://doi.org/10.1007/s12032-017-0969-3.
- Gonzalez-Reyes, C.; Marcial-Medina, C.; Cervantes-Anaya, N.; Cortes-Reynosa, P.; Salazar, E.P. Migration and invasion induced by linoleic acid are mediated through fascin in MDA-MB-231 breast cancer cells. Cell. Biochem. 2017, 443, 1–10. https://doi.org/10.1007/s11010-017-3205-8.
- Cao, Q.; Hersl, J.; La, H.; Smith, M.; Jenkins, J.; Goloubeva, O.; Dilsizian, V.; Tkaczuk, K.; Chen, W.; Jones, L. A pilot study of FDG PET/CT detects a link between brown adipose tissue and breast cancer. BMC Cancer 2014, 14, 126. https://doi.org/10.1186/1471-2407-14-126.
- Villegas-Comonfort, S.; Castillo-Sanchez, R.; Serna-Marquez, N.; Cortes-Reynosa, P.; Salazar, E.P. Arachidonic acid promotes migration and invasion through a PI3K/Akt-dependent pathway in MDA-MB-231 breast cancer cells. Prostaglandins, Leukot. Essent. Fat. Acids 2014, 90, 169–177. https://doi.org/10.1016/j.plefa.2014.01.007.
- Naughton, S.S.; Mathai, M.L.; Hryciw, D.H.; McAinch, A.J. Linoleic acid and the pathogenesis of obesity. Prostaglandins Other Lipid Mediat. 2016, 125, 90–99. https://doi.org/10.1016/j.prostaglandins.2016.06.003.
- Liu, J.; Han, L.; Zhu, L.; Yu, Y. Free fatty acids, not triglycerides, are associated with non-alcoholic liver injury progression in high fat diet induced obese rats. Lipids Health Dis. 2016, 15, 1–9. https://doi.org/10.1186/s12944-016-0194-7.
- Zhang, J.; Zhao, Y.; Xu, C.; Hong, Y.; Lu, H.; Wu, J.; Chen, Y. Association between serum free fatty acid levels and nonalcoholic fatty liver disease: A cross-sectional study. Rep. 2014, 4, srep05832. https://doi.org/10.1038/srep05832.
- Schuster, S.; Johnson, C.D.; Hennebelle, M.; Holtmann, T.; Taha, A.Y.; Kirpich, I.A.; Eguchi, A.; Ramsden, C.E.; Papouchado, B.G.; McClain, C.J.; et al. Oxidized linoleic acid metabolites induce liver mitochondrial dysfunction, apoptosis, and NLRP3 activation in mice. Lipid Res. 2018, 59, 1597–1609. https://doi.org/10.1194/jlr.m083741.
- Hillers-Ziemer, L.E.; Kuziel, G.; Williams, A.E.; Moore, B.N.; Arendt, L.M. Breast cancer microenvironment and obesity: Challenges for therapy. Cancer Metastasis Rev. 2022, 41, 627–647. https://doi.org/10.1007/s10555-022-10031-9.
- Kim, D.-G.; Krenz, A.; Toussaint, L.E.; Maurer, K.J.; Robinson, S.-A.; Yan, A.; Torres, L.; Bynoe, M.S. Non-alcoholic fatty liver disease induces signs of Alzheimer’s disease (AD) in wild-type mice and accelerates pathological signs of AD in an AD model. Neuroinflammation 2016, 13, 1–18. https://doi.org/10.1186/s12974-015-0467-5.
- Zhang, C.; Duan, J.; Yang, H.; Sun, C.; Zhong, W.; Tao, J.; Guan, X.; Jiang, H.; Hammock, B.D.; Hwang, S.H.; et al. COX‐2/sEH dual inhibitor PTUPB alleviates bleomycin‐induced pulmonary fibrosis in mice via inhibiting senescence. FEBS J. 2019, 287, 1666–1680. https://doi.org/10.1111/febs.15105.
- Lee, Y.-S.; Lee, H.S.; Chang, S.W.; Lee, C.U.; Kim, J.S.; Jung, Y.K.; Kim, J.H.; Seo, Y.S.; Yim, H.J.; Lee, C.H.; et al. Underlying nonalcoholic fatty liver disease is a significant factor for breast cancer recurrence after curative surgery. Medicine 2019, 98, e17277. https://doi.org/10.1097/md.0000000000017277.
- Bilici, A.; Ozguroglu, M.; Mihmanlı, I.; Turna, H.; Adaletli, I. A case–control study of non-alcoholic fatty liver disease in breast cancer. Med Oncol. 2007, 24, 367–371. https://doi.org/10.1007/s12032-007-0034-8.
- Esquivel-Velázquez, M.; Ostoa-Saloma, P.; Palacios-Arreola, M.I.; Nava-Castro, K.E.; Castro, J.I.; Morales-Montor, J. The Role of Cytokines in Breast Cancer Development and Progression. Interf. Cytokine Res. 2015, 35, 1–16. https://doi.org/10.1089/jir.2014.0026.
- Lengacher, C.A.; Reich, R.R.; Paterson, C.L.; Shelton, M.; Shivers, S.; Ramesar, S.; Pleasant, M.L.; Budhrani-Shani, P.; Groer, M.; Post-White, J.; et al. A Large Randomized Trial: Effects of Mindfulness-Based Stress Reduction (MBSR) for Breast Cancer (BC) Survivors on Salivary Cortisol and IL-6. Res. Nurs. 2018, 21, 39–49. https://doi.org/10.1177/1099800418789777.
- Danforth, D. The Role of Chronic Inflammation in the Development of Breast Cancer. Cancers 2021, 13, 3918. https://doi.org/10.3390/cancers13153918.
- Ruffell, B.; Au, A.; Rugo, H.S.; Esserman, L.J.; Hwang, E.S.; Coussens, L.M. Leukocyte composition of human breast cancer. Natl. Acad. Sci. USA 2011, 109, 2796–2801. https://doi.org/10.1073/pnas.1104303108.
- Wang, J.; Cai, D.; Ma, B.; Wu, G.; Wu, J. Skewing the Balance of Regulatory T-Cells and T-Helper 17 Cells in Breast Cancer Patients. Int. Med. Res. 2011, 39, 691–701. https://doi.org/10.1177/147323001103900301.
- Mohammed, Z.M.A.; Going, J.; Edwards, J.; Elsberger, B.; Doughty, J.C.; McMillan, D. The relationship between components of tumour inflammatory cell infiltrate and clinicopathological factors and survival in patients with primary operable invasive ductal breast cancer. Br. J. Cancer 2012, 107, 864–873. https://doi.org/10.1038/bjc.2012.347.
This entry is adapted from the peer-reviewed paper 10.3390/molecules27217269
This entry is offline, you can click here to edit this entry!
