Antibodies (Abs) are important immune mediators and powerful diagnostic markers in a wide range of infectious diseases. Understanding the humoral immunity or the development of effective antibodies against SARS-CoV-2 is a prerequisite for limiting disease burden in the community and aids in the development of new diagnostic, therapeutic, and vaccination options. Antibody testing showed the potential in adding important diagnostic value to the routine diagnosis and clinical management of COVID-19. They could also play a critical role in COVID-19 surveillance, allowing for a better understanding of the full scope of the disease. The development of several vaccines and the success of passive immunotherapy suggest that anti-SARS-CoV-2 antibodies have the potential to be used in the treatment and prevention of SARS-CoV-2 infection.
1. Introduction
The persisting spreading of SARS-CoV-2 has prompted the scientific community to develop effective vaccine candidates and to produce or find potential drugs or passive immune strategies. The scientific efforts have also focused on acquiring rapid and accurate SARS-CoV-2 diagnostic tests, which are critical for developing effective COVID-19 containment strategies. The human immune response remains the most effective mechanism of combating SARS-CoV-2 infection [
4]. Despite the fact that both innate and adaptive immunity are important, SARS-CoV-2-specific humoral immunity has proven to be crucial in determining the disease outcome [
5]. Understanding the humoral immunity—or the development of antibodies against SARS-CoV-2—is a prerequisite for limiting disease burden in the community and aids in the development of new diagnostic, therapeutic, and vaccination options [
5].
The insufficient testing capacity of the real-time reverse transcriptase polymerase chain reaction (qRT-PCR), particularly in low-resource countries, has highlighted the need for an alternative rapid, simple, accurate, and relatively inexpensive diagnostic approach. For diagnostic purposes, anti-SARS-CoV-2 antibodies represent the most easily identifiable targets [
6]. As of now, serological tests have been substantially considered for use as a complements or alternatives to qRT-PCR. Thus, a number of SARS-CoV-2 serodiagnostic tests have been developed and assessed. Many of these tests have proven valuable in detecting SARS-CoV-2 antigens and/or antibodies. Antibody tests have the potential to add important diagnostic value to the routine diagnosis and clinical management of COVID-19. They could also play a critical role in SARS-CoV-2 surveillance for understanding the full scope of the disease and to rebuild public confidence.
While the rapid development of many SARS-CoV-2 vaccines is an extraordinary achievement, the continual emergence of new SARS-CoV-2 variants raises additional questions about the capability of the new virus variants to alter the efficacy of the current vaccine candidates. Therefore, data on the antibody dynamics of SARS-CoV-2-infected individuals or on the vaccination-induced immune responses are critical for understanding vaccine protection and durability, as well as for determining whether additional booster doses are required.
2. Key Proteins of SARS-CoV-2
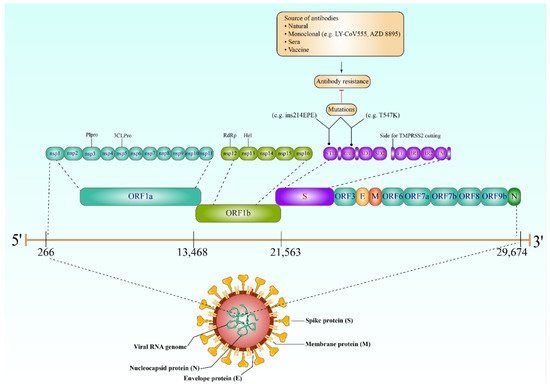
Understanding the behavior of SARS-CoV-2 key proteins is crucial for developing diagnostic tests, vaccines, and therapeutics. The genome of SARS-CoV-2 encodes both structural and nonstructural proteins. Spike (S), nucleocapsid (N), membrane (M), and envelope (E) are the four key structural proteins that have the potential to be targeted by the antibody response (
Figure 1) [
9]. The choice of antigen is crucially important in utilizing the virus-specific antibodies for detecting SARS-CoV-2 infection and aiding the development of therapeutics and vaccine candidates. Achieving high sensitivity and specificity when developing antibody tests is mainly dependent on the selection of the diagnostic antigens [
10]. However, focusing on the proteins required for viral entry would have a significant impact on the development of any therapeutics or vaccination strategies; the E and M proteins have an essential role in viral assembly [
11], and the N protein has been proved to be necessary for viral RNA synthesis (
Figure 1) [
12].
Figure 1. The SARS-CoV-2 genome, expected mutations (antibody resistance), and the structural proteins. The genome virus contains ORF1a (nsp1, 2, 3 (Plpro), 4, 5 (3CLPro), 6, 7, 8, 9, 10, and 11), ORF1b (nsp12 (RdRp), 13 (Hel), 14, 15, and 16), S (NTD, RBD, SD1/2, FL, HR1/2, and TM), ORF3, E, M, ORF6, ORF7a/b, ORF8, ORF9, and N, respectively. The complete virus also contains four structural proteins: Spike (S), nucleocapsid (N), membrane (M), and envelope (E) proteins, in addition to the viral RNA genome. Interestingly, mutations (e.g., T547 and ins214EPE) in the viral S gene (which produces the spike proteins, specifically NTD and RBD) have made the SARS-CoV-2 highly resistant to a wide range of antibodies, including monoclonal, those from natural infection or sera, and, finally, those produced after vaccination. RdRp, RNA-dependent RNA polymerase; RBD, receptor-binding domain; Plpro, papain-like protease; NTD, N-terminal domain; 3CLPro, 3C-like proteinase; SD1, subdomain 1, SD2 subdomain 2; Hel, helicase; HR1, heptad repeat 1; HR2, heptad repeat 2; TM, transmembrane domain; FL, fusion loop.
3. Antibody Response against SARS-CoV-2
Similar to all viral infections, virus-specific antibodies are crucial for the recognition of, clearance of, and protection against SARS-CoV-2. Antibody response to SARS-CoV-2 infection first develops against the N protein. However, protective immunity against SARS-CoV-2 infection is mostly dependent on the neutralizing antibody responses that target the virus’s S protein. Studies on the time and durability of the neutralizing antibody production following SARS-CoV-2 infection revealed that patients begin to generate these antibodies by week two, and the majority of them develop neutralizing antibodies by week three. However, in most recovered patients, independent of age or comorbidities, neutralizing antibody titers gradually declined after 5–8 weeks but continued to be detectable for up to eight months [
16].
The transition from a seronegative to a seropositive condition or the development of detectable antibodies in a patient’s serum is known as seroconversion [
17]. Many studies have analyzed the kinetics of antibody response in COVID-19 patients. Both severe and nonsevere patients have reported stronger total antibodies as well as single Ig classes’ responses (IgM, IgA, and IgG).
4. The Role of Antibodies in SARS-CoV-2 Diagnosis
4.1. COVID-19 Antibody Tests
Unlike molecular techniques, antibody tests (also known as serological assays) rely on the detection of either viral antigenic proteins or diagnostically detectable antibodies which are created during the immune response to SARS-CoV-2 infection [
27]. The main advantage of SARS-CoV-2 antigen tests is that the results—detecting viral antigens in throat or nasal swab samples—can be obtained in minutes [
28]. For this reason, using antigen tests is practical, particularly for a large number of people. However, the gradual decline in viral load over time may cause difficulty in detecting viral antigens [
29]. Instead, measuring the antibodies produced during SARS-CoV-2 infection has shown great potential for the indirect detection of the virus in a larger time window. In addition, antibody testing can play a crucial role in SARS-CoV-2 contact tracing, surveillance, and epidemiological efforts [
30].
Many serological assays have been developed to detect SARS-CoV-2-specific IgM, IgG, and IgA, despite the uncertainty about using these different isotypes individually or in combination [
31]. However, none of these antibody isotypes have been clearly identified as the optimal option in the scenario of COVID-19, even though the accurate interpretation of serodiagnostic tests currently depends on the type of antibodies being detected. Indeed, many serological platforms have been utilized to measure the presence of SARS-CoV-2 antibodies and/or antigens. These platforms generally take the form of chemiluminescence immunoassays (CLIA) [
32,
33], enzyme-linked immunosorbent assay (ELISA) [
21,
34], fluorescence immunoassays (FIA), rapid diagnostic tests (RDTs) and neutralization assays [
35,
36].
4.2. Implications of Seroconversion in SARS-CoV-2 Antibody Tests
The time required by the host immune system to develop an antibody response significantly affects the capability of the serological tests to confirm SARS-CoV infection immediately after a person contracts the virus or during the early stage of infection [
56]. Understanding the timing of seroconversion is crucial in determining the optimal time points for specimen collection [
57]. Accordingly, establishing or planning any diagnostic protocol involving antibody tests should consider seroconversion time because it has a pivotal role in the efficacy of serodiagnostic tests.
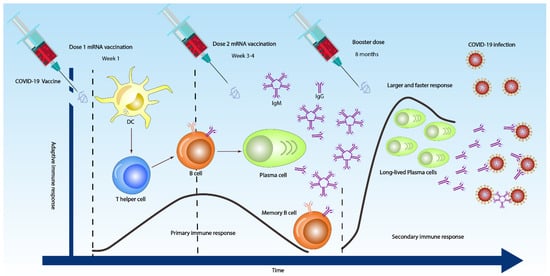
Similar to patients with SARS-CoV, the majority of COVID-19 patients seroconvert after seven days of contracting the virus. The median seroconversion times for IgM and IgG were 12 and 14 days, respectively (
Figure 2) [
29,
58,
59]. Even though in some patients, IgM and IgG were detected as early as four or five days (
Figure 2) [
60,
61].
Figure 2. Overall antibody responses to 3 COVID-19 doses. After the first dose (sometimes IgM and IgG are detected as early as day 4 or 5 in week one), the dendritic cell (DC) in the body activates both T cells to differentiate into T helper 2 (Th2) cells and naïve B cell to differentiate into plasma cells. After the second dose at 3–4 weeks, the immune response becomes stronger and faster (primary immune response), producing high IgM and low IgG antibodies. However, booster vaccine injection might induce a very strong and vigorous immune response, with long-lived plasma cells producing low IgM and high IgG antibodies that may effectively opsonize, neutralizing the SARS-CoV-2 viruses (secondary immune response).
4.3. Antibody Tests at an Individual Level
Given the uncertainties about the accuracy of antibody tests, additional information is required to support recommending serological testing for use as the sole basis to confirm or exclude active SARS-CoV-2 infection [
14]. Nonetheless, and despite critical knowledge gaps, the use of antibody tests in the clinical diagnosis of COVID-19 and assessing immunity to the virus is critical [
66]. Given the limited availability of molecular testing, particularly in limited-resource countries, it is necessary to prioritize who gets tested. Accordingly, the WHO, the center for disease prevention and control (CDC), and the European center for disease prevention and control (ECDC) have suggested specific criteria for prioritizing testing. Under this scenario, antigen and antibody tests may offer another avenue to be used as an alternative for diagnosing those not prioritized for molecular testing with specific caution. In general, antibody tests may be utilized at an individual level as a complement to molecular testing for investigating patients with negative qRT-PCR results [
26,
61]. It may be used to monitor and identify asymptomatic infections amongst close contacts [
17]. It may help to understand a patient’s clinical findings, particularly in clinically complicated COVID-19 cases, if multisystem inflammatory syndromes are detected [
67]. Antibody tests can be further applied to determine who is qualified to donate convalescent plasma and to screen COVID-19 survivors who undergo convalescent plasma treatments to determine whether they develop their own immunity [
68]. Thus, antibody tests may eventually be useful in studying the kinetics of SARS-CoV-2 antibody responses [
21]. Serological measurements at baseline, as well as during and after immunization trials, are important for evaluating any vaccine candidate.
A single negative serological test may reflect a false-negative result, so it does not exclude SARS-CoV-2 infection [
66,
70], particularly in highly exposed persons; this could be due to the low antibody concentrations if the test is performed at the beginning of the infection [
68,
71], the type of a specimen, or due to the decrease in the number of antibodies after the clearance of the infection. In this case, repeating the test is the best advice. The relatively low negative predictive value of many COVID-19 antibody tests indicates the missing data of many acute infections based on seronegative results [
26]. Negative results also allow clinicians to suspect other diseases, because COVID-19 symptoms can resemble those of many other diseases.
By contrast, a positive antigen-based detection test is considered very accurate for identifying acute or early infection [
72] and can indicate that a person is likely infected with SARS-CoV-2. Nevertheless, the test positivity may be due to cross-reactivity with other infections, including other human coronaviruses [
73]. Therefore, a full-panel test, including other CoVs, SARS-CoV-2, bacterial bronchitis, and influenza, is recommended if applicable [
74].
4.4. Population Serological Testing
Serological testing can help identify who is infected or exposed and who is immune by assuming protective immunity. Accordingly, population-based serological information will be helpful for officials in making decisions about lifting or enforcing any control measures. The potential of this test in determining the accurate number of infected people in a large population has been tested [83,84]. Population seropositivity indicates that the number of people positive for anti-SARS-CoV-2 antibodies is much higher than that of the reported cases [84,85]. Population-based serological surveillance has been carried out around the world, including countries in Europe [86,87], America [88,89], Asia [90,91], and Africa [92,93]. Indeed, healthcare workers are the population most targeted for serological surveillance due to their high risk of SARS-CoV-2 infection, with ELISA being the most commonly used diagnostic tool for detecting anti-SARS-CoV-2 antibodies.
5. Antibody Tests and Seroprotection
5.1. Antibodies in SARS-CoV-2 Vaccine Development
Immunity against SARS-CoV-2 infection is normally acquired in one of two ways. Contracting the virus typically ends in natural immunity for a certain period, and vaccination is another way to become resistant. The entry of SARS-CoV-2 into the human cell is the first step of the infection and one of the most crucial processes in the virus’ life cycle. As a result, it is a prime target for vaccinations and therapeutics.
The virus enters the cells of the lung, gastrointestinal tract (GI tract), kidneys, liver, heart, and other organs through the binding of the S protein’s receptor-binding domain (RBD) to its target host receptor, the angiotensin-converting enzyme 2 (ACE2), and a host protease known as transmembrane serine protease 2 (TMPRSS2), which facilitates the cleavage of the S glycoprotein, allowing viral access into the host cells [
99,
100]. Therefore, one of the main goals of SARS-CoV-2 vaccine development is to generate neutralizing antibodies that block virus entry or prevent membrane fusion.
Despite the fact that the post-vaccination immune response has several components, including innate, humoral, cellular, and cytokine responses, immunological surveillance that measures antibody response is fundamental for assessing the efficacy of all SARS-CoV-2 vaccines. In particular, measuring the level of circulating anti-S-RBD antibodies could provide important information on SARS-CoV-2 acquired immunity [
102].
5.2. Mechanism of Antibody-Mediated Protection
The role of antibodies in resistance to SARS-CoV-2 infection was explored. Understanding the properties and mechanisms by which antibodies provide protection is essential to defining immunity. Infection or vaccination history may have a role in providing protection against the subsequent infection, and several studies have provided evidence for such protective associations [
105]. Upon infection, pre-existing antibodies bind to the surface of SARS-CoV-2 virus particles and lead to the neutralization of the viral spike. Neutralizing antibodies are critical for the efficacy of any SARS-CoV-2 vaccine. Over the past two years, SARS-CoV-2-neutralizing antibodies have been developed for preventive or therapeutic uses [
106,
107,
108]. Most of the neutralizing antibodies target the S protein; their neutralization potency and breadth vary according to recognition epitopes. These findings have prompted an intense effort to identify potential immunodominant epitopes that are recognized by broadly neutralizing the antibodies that could be used as templates for SARS-CoV-2 vaccine design.
5.3. Prevaccination Antibody Screening
Notably, limited-resource countries (LRCs) remain at the back of the line when it comes to new technologies, infrastructure, and public health control measures such as vaccines. Evidently, COVID-19 mass vaccination is currently not applicable in most of the LRCs, where a lack of resources is placing enormous pressure on governments to accelerate the mass vaccination strategies. Accordingly, the establishment of new strategies that maximize the number of individuals who get vaccines without losing the efficacy of immune protection is urgently needed. The limited availability of authorized SARS-CoV-2 vaccines has led to widespread consideration of a single vaccine dose for people with past SARS-CoV-2 infection [
110].
6. Resistance to SARS-CoV-2 Antibodies
Resistance to the SARS-CoV-2’s antibody is now a living fact due to the introduction of new virus variants, and this places an enormous load on the vaccination process. As such, recent vaccines have been developed to contradict the virus that was first discovered in late 2019 in Wuhan, China [
116]. However, emerged variants such as the South African-Beta (B.1.351) and the UK-Alpha (B.1.1.7) have demonstrated extensive mutations in their S proteins, and these variants have been demonstrated to be highly contiguous [
117]. Indeed, almost all monoclonal antibodies directed against the S protein’s N-terminal domain failed to recognize Alpha, although antibodies directed against the receptor-binding region were more effective [
118]. Nonetheless, the variation exhibited reduced affinity for plasma from patients who have recovered from SARS-CoV-2 or sera from those who have been immunized against SARS-CoV-2. Both monoclonal antibodies directed against the N-terminal domain and several separate monoclonal antibodies directed against the receptor-binding motif are very ineffective against the mutated Beta, and this resistance might have been acquired from the mutated E484K substitution. Furthermore, Beta is much more resistant to neutralization by convalescent plasma (9.4-fold) and serum (10.3–12.4-fold) from BNT162b2-immunized individuals [
118]. Furthermore, the Beta SARS-CoV-2 variants, which had various changes in their S proteins, were resistant to 17 neutralizing monoclonal antibodies as well as sera from convalescent patients and vaccinated mice, which were unable to neutralize the variants [
119].
Subsequent studies indicated that certain Omicron variants are now resistant to antibodies elicited by vaccine doses. Remarkably, Omicron S proteins evaded blockage by antibodies obtained from persons inoculated with the BioNTech-Pfizer vaccine (BNT162b2) or convalescent patients with 12- to 44-fold more efficiency than the Delta (B.1.617.2) variant S protein [
120].
Furthermore, the antibodies ReGN10933, REGN10987, and JS016 and serum from vaccinated participants neutralized the S protein of numerous wild-type variations, including Alpha, Beta, Gamma, and Delta. Regn10987 antibodies, for example, were the most effective in neutralizing the aforementioned variations. In addition, the Regn10933 and JS016 antibodies were both effective, although their reactions to the S protein were quite different [
125]. These two antibodies, however, were unable to neutralize the Beta version. Unexpectedly, none of these antibodies neutralized the Omicron variant [
125]. This result implies that these antibodies cannot be used to combat the ongoing Omicron variant pandemic or any developing variation.
Despite immunocompromised individuals potentially being more susceptible to SARS-CoV-2 viruses with unusual manifestations, prolonged immunosuppression treatment may provide some defense from severe COVID-19 disease consequences. However, the possibility of immunocompromised people acquiring abnormally severe COVID-19 is currently elusive [
126]. Recent evidence shows that immunocompromised people may benefit greatly from convalescent plasma therapy [
127,
128,
129,
130], and that mutations and newly emerged virus strains will lead to more severe complications in the group. New variants, however, can appear in this patient population as a result of the selection pressure brought on by a severe viral infection [
131]. The majority of people who have severe SARS-CoV-2 have immune system issues, thus the virus may live on for a very long period. Patients with impaired immune systems have been demonstrated to have varying SARS-CoV-2 evolution patterns [
131]. How selection forces and evolutionary processes interact during chronic infection is an issue that has yet to be resolved. With that in mind, due to mutations (Q493K
RBD, Q493K/R
RBD) in the S protein in immunocompromised individuals, antibodies that were recovered from a healthy COVID-19 convalescent donor were shown to be ineffective in providing protection against SARS-CoV-2. In particular, the Q493K
RBD mutation 15-fold reduced the effectiveness of REGN10933 pseudotype neutralization, but the Q493K/R
RBD mutations almost completely imparted resistance to healthy COVID-19 convalescent donor IgG [
132].
7. Limitations of Antibody Tests
Although antibody tests are useful in COVID-19 case management and in vaccinations effectiveness, a number of drawbacks occur. The significant limitation is that antibodies may be present at undetectable levels in early days, thereby influencing the potency of any serodiagnostic tests and their effectiveness for diagnosing SARS-CoV-2 infection [
135,
136]. In this situation, a false-negative serological result from individuals with replicating and shedding viruses can have serious public health consequences [
68]. Another limitation is the unknown duration at which IgM or IgG antibodies remain detectable after the virus has been cleared from the body. Furthermore, variations in antigens and methodologies used in IgM and IgG detection kits are essential, and they affect the sensitivity and specificity of the tests [
137]. Unfortunately, the inaccuracy of antibody tests is unavoidable and will inevitably lead to false-positive or -negative results and disease misclassifications, particularly if these tests are not properly conducted and interpreted [
138]. Moreover, the proven cross-reactivity of SARS-CoV-2 antibody tests with other coronaviruses is difficult to avoid [
73]. Indeed, increasing the levels and duration of antibodies after SARS-CoV-2 vaccination to provide full protection and diagnostic opportunity against the current variants and those that can emerge in the future is still the primary objective in the upcoming studies.
This entry is adapted from the peer-reviewed paper 10.3390/vaccines10081346