TiO2 probably plays the most important role in photocatalysis due to its excellent chemical and physical properties. However, the band gap of TiO2 corresponds to the Ultraviolet (UV) region, which is inactive under visible irradiation. At present, TiO2 has become activated in the visible light region by metal and nonmetal doping and the fabrication of composites. Recently, nano-TiO2 has attracted much attention due to its characteristics of larger specific surface area and more exposed surface active sites. nano-TiO2 has been obtained in many morphologies such as ultrathin nanosheets, nanotubes, and hollow nanospheres.
- nano-TiO2
- photocatalytic applications
- visible light
- doping
- vacancy
- composite
1. Introduction
In nature, TiO2 usually has three different crystal structures: anatase, rutile, and brookite [1]. In addition, there are several metastable crystal structures of TiO2 such as TiO2 (H) and TiO2II [2]. These metastable crystal structures can be obtained by artificial synthesis. Rutile is the most stable crystal form of TiO2. Even when the particle size is reduced to the nanometer level, rutile is still the most stable TiO2 nanomaterial. Anatase and brookite can be transformed into rutile at high temperature. Different crystal types of TiO2 usually exhibit different morphologies and properties. Therefore, the synthesis methods and conditions for different crystal types of TiO2 nanomaterials are also different. For example, the synthesis of anatase TiO2 nanomaterials usually requires solution synthesis or low temperature chemical vapor deposition, however, the synthesis of rutile TiO2 nanomaterials requires high temperature deposition or heating reaction [3].
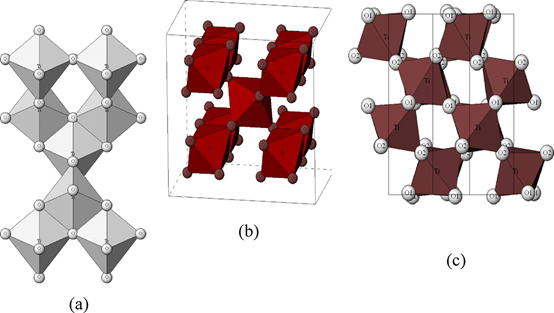
Figure 1 is the crystal structure of three different TiO2 phases, and the differences in crystal structures are quite evident. Rutile TiO2 has a tetragonal structure (Figure 1b), and its {011} and {100} crystal facets have the lowest energy, therefore, its thermodynamically stable morphology is a truncated octahedron. Anatase has a tetragonal structure, and its c-axis is longer than the a-axis (Figure 1a). Anatase TiO2 also has a low energy crystal plane, which is the same as rutile, so it can show as a truncated octahedron. The brookite belongs to an orthorhombic structure, and its structural unit is relatively larger, which is composed of eight TiO2 units (Figure 1c).
Figure 1. Crystalline structures of titanium dioxide (a) anatase, (b) rutile, and (c) brookite. Reprinted with permission from Advanced Industrial Science and Technology (AIST) https://staff.aist.go.jp/nomura-k/english/itscgallary-e.htm.
Jinfeng Zhang et al. [4] calculated the electronic structure and the effective mass of the carrier for anatase, rutile, and brookite TiO2 by using the plane-wave pseudopotential method, to prove that anatase has higher photocatalytic performance than rutile and brookite. The calculation results showed that rutile had the narrowest band gap of 1.86 eV, and the band gaps of anatase and brookite were 2.13 and 2.38 eV, respectively. However, anatase is an indirect band gap semiconductor, and rutile and brookite both belong to the direct band gap semiconductor. Therefore, this leads to longer lifetimes of photogenerated electrons and holes for anatase than those for rutile and brookite. The valence bands of TiO2 in anatase, rutile, and brookite are mainly composed of O 2p and mixed with a few Ti 3d. Above the Fermi level, the conduction band is composed of Ti 3d, mixed with a small amount of O 2p and Ti 3p. The calculation results show that the anatase has a smaller average effective mass of photogenerated electrons and holes than rutile and brookite. The smaller the effective mass of the photogenerated electrons and holes, the easier it is for them to migrate, thus improving the photocatalytic activity. As anatase has a smaller effective mass and a longer lifetime of photogenerated electrons and holes, in general, anatase TiO2 has a higher photocatalytic activity.
The energy level structure of semiconductor material contains two aspects: energy level position and energy band width. The position of its energy level determines whether the photocatalytic reaction can take place, and the energy band width determines its light absorption range. The position of the titanium dioxide energy level is decisive for the photocatalytic reaction. From a thermodynamic point of view, when the reduction potential of the reactant is lower than the conduction band of the semiconductor material, a reduction reaction can occur; whereas when the oxidation potential is higher than the valence band of the semiconductor material, an oxidation reaction can occur [5]. Taking the photolysis of water as an example, the generation of H2 is the process of reducing H+ by photogenerated electrons, while the generation of O2 molecules is the process of oxidizing O2− by holes. The energy band position of TiO2 is suitable for the photolysis of water, because the valence band of TiO2 (+2.7 V, pH = 7) is lower than the redox potential of O2/H2O (+1.23 V, pH = 7) and the position of its conduction band (−0.5 V, pH = 7) is higher than the position of H2O/H2 redox potential (−0.41 V, pH = 7) [6].
In addition to the position of the energy level, the band width also has a very important effect on photocatalytic performance. For example, the band width (3.2 eV) of TiO2 is wide, so it can only absorb ultraviolet light. It is only possible to use visible light when TiO2 is doped with some metals, non-metallic elements, or combined with other semiconductors with smaller energy band widths. For example, g–C3N4 has a moderate forbidden band width (2.7 eV), and its conduction band position is very high (−1.3 V, pH = 7) [7]. Therefore, the combination of TiO2 and g–C3N4 can improve the utilization of visible light.
2. Nano-TiO2 Morphology
A large number of studies have shown that the morphology of nanomaterials has a very important effect on photocatalytic performance, because the morphology usually determines the exposure of the crystal plane and active site, specific surface area, electron, and hole transport rate and other factors.
Zero-dimensional TiO2 nanomaterial has an isotropic structure and can expose all crystal planes (including those with higher energy), which is conducive to photocatalytic reactions. However, due to the quantum confinement effect, it has a larger forbidden band width and more surface defect states, making the photogenerated electrons and holes to have a higher recombination efficiency. If the surface can be properly modified, the recombination efficiency of electron-hole pairs can be greatly reduced, which is conducive to improving the photocatalytic performance of zero-dimensional TiO2 nanomaterials. This improved method has also been applied to many other zero-dimensional semiconductor nanomaterials such as carbon dots [8][9], CdS [10], CdSe [11], and graphene quantum dots [12][13].
The one-dimensional structure of TiO2 such as nanorods, nanowires, and nanotubes possesses a very fast charge transfer rate in a single direction, and the electron-hole pair has a relatively low recombination efficiency, making it an important research object for photocatalytic reactions [14][15].
Two-dimensional TiO2 nanosheets are very thin, with large specific surface area and effective absorption area, and the rate of charge transfer is also very fast. Therefore, two-dimensional sheet TiO2 material is also widely used in photocatalysis [16][17].
In recent years, hierarchical structure TiO2 nanomaterials composed of multiple morphologies have also been used in photocatalytic reactions [18][19]. These hierarchical structures of TiO2 can simultaneously combine the advantages of different structures and effectively improve their photocatalytic performance.
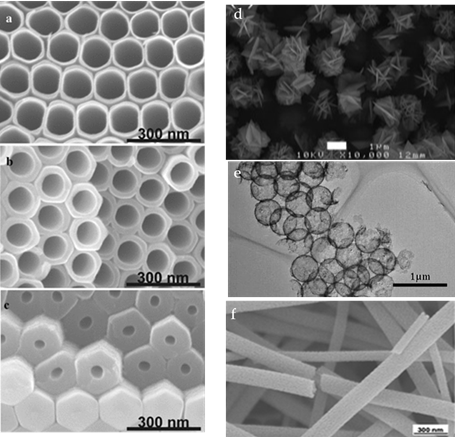
Macak et al. [20] successfully prepared idealized TiO2 nanotubes by anodizing a Ti substrate with a glycol electrolyte containing NH4F and exploring the oxidation conditions (Figure 2a–c). The presence of hexagonal nanotubes can be clearly seen from the entire layer, arranged in neat rows, with each nanotube remaining hexagonal from top to bottom. The lower wall thickness was about 65 nm and the upper wall thickness was about 12 nm. The diameter of the internal pipe increased gradually from about 50 nm to 110 nm.
Fang et al. [21] reported a new synthesis method for TiO2 nanometer flowers with a large amount of {001} crystal surface exposed (Figure 2d). These nanometer flowers were completely assembled from TiO2 nanometer flakes with a size of about 2.0 μm, with a thickness of about 10–20 nm and a length of about 1.2 μm.
The submicron scale hollow sphere of TiO2 not only has a large specific surface, but also has a size near the wavelength of UV–Vis. Therefore, in theory, diffraction and reflection caused by shell structure on the hollow sphere can improve the utilization rate of light [22]. In the presence of cationic polystyrene sphere (PS) templates, Yoshihiko Kondo et al. [23] prepared submicron hollow sphere TiO2 by hydrolyzing isopropyl titanate (Figure 2e). Uniform anatase TiO2 hollow pellets with a diameter of about 490 nm and a shell thickness of about 30 nm were obtained. The resulting surface area measured by Brunauer–Emmett–Teller was 70 m2/g. The photocatalytic properties were tested by the decomposition of isopropanol under ultraviolet light.
Shuai Chen et al. [24] prepared anatase TiO2 nanorods by electrospinning and roasting. As shown in Figure 2f, the nanorods were observed to be 200 nm to 2 μm in length and 60 nm to 150 nm in diameter. The electrical properties of TiO2 nanobelts on curved surfaces with different curvature and their photoelectric properties under different light intensities were studied. The results showed that TiO2 nanobelts have potential applications in flexible photodetectors and solar cells.
Figure 2. Scanning Electron Microscope(SEM) images of TiO2 nanotubes taken from the upper part of the layer (a), the middle of the layer (b), and the bottom of the layer (c), reprinted with permission from [70]. (d) SEM images of flower TiO2 reprinted with permission from [25], (e) Transmission Electron Microscope (TEM) image of TiO2 hollow spheres reprinted with permission from [21]. (f) SEM images of TiO2 nanobelts reprinted with permission from [23].
3. Conclusions
Due to its physical structure and good optical properties, titanium dioxide is considered to be a promising semiconductor photocatalyst, while nano-TiO2 has the advantages of large specific surface area and more exposed active sites, so it has better performance than TiO2. The important environmental applications of the nano-TiO2 photocatalyst were highlighted in this review such as hydrogen production, dye degradation, CO2 degradation, and nitrogen fixation. As reviewed here, a number of studies focused on making nano-TiO2 active in the visible light region by various methods such as doping of metals or nonmetals, manufacturing defects, and compounding of other semiconductors. So far, the successful application of nano-TiO2 photocatalysts in visible light has only been on a laboratory scale. Future research should focus on the use of novel nano-TiO2 photocatalysts (doped nano-TiO2 or composite nano-TiO2) for large-scale application.
References
- Nolan, N.T.; Seery, M.K.; Pillai, S.C. Spectroscopic Investigation of the Anatase-to-Rutile Transformation of Sol−Gel-Synthesized TiO2 Photocatalysts. J. Phys. Chem. C 2009, 113, 16151–16157.
- Curnan, M.T.; Kitchin, J.R. Investigating the Energetic Ordering of Stable and Metastable TiO2 Polymorphs Using DFT+U and Hybrid Functionals. J. Phys. Chem. C 2015, 119, 21060–21071.
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285.
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. PCCP 2014, 16, 20382–20386.
- Tahir, M.; Amin, N.S. Advances in visible light responsive titanium oxide-based photocatalysts for CO2 conversion to hydrocarbon fuels. Energy Convers. Manag. 2013, 76, 194–214.
- Chen, W.-T.; Chan, A.; Al-Azri, Z.H.N.; Dosado, A.G.; Nadeem, M.A.; Sun-Waterhouse, D.; Idriss, H.; Waterhouse, G.I.N. Effect of TiO2 polymorph and alcohol sacrificial agent on the activity of Au/TiO2 photocatalysts for H2 production in alcohol–water mixtures. J. Catal. 2015, 329, 499–513.
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123.
- Zhu, C.; Liu, C.; Zhou, Y.; Fu, Y.; Guo, S.; Li, H.; Zhao, S.; Huang, H.; Liu, Y.; Kang, Z. Carbon dots enhance the stability of CdS for visible-light-driven overall water splitting. Appl. Catal. B 2017, 216, 114–121.
- Sang, L.; Lin, J.; Zhao, Y. Preparation of carbon dots/TiO2 electrodes and their photoelectrochemical activities for water splitting. Int. J. Hydrogen Energy 2017, 42, 12122–12132.
- Lin, C.-J.; Kao, L.-C.; Huang, Y.; Bañares, M.A.; Liou, S.Y.-H. Uniform deposition of coupled CdS and CdSe quantum dots on ZnO nanorod arrays as electrodes for photoelectrochemical solar water splitting. Int. J. Hydrogen Energy 2015, 40, 1388–1393.
- Kang, S.H.; Lee, S.-Y.; Gang, M.G.; Ahn, K.-S.; Kim, J.H. Bifunctional Effects of CdSe Quantum Dots and Nb2O5 Interlayer for ZnO Nanorods-based Photoelectrochemical Water-Splitting Cells. Electrochim. Acta 2014, 133, 262–267.
- Azimirad, R.; Safa, S.; Ebrahimi, M.; Yousefzadeh, S.; Moshfegh, A.Z. Photoelectrochemical activity of graphene quantum dots/hierarchical porous TiO2 photoanode. J. Alloys Compd. 2017, 721, 36–44.
- Majumder, T.; Mondal, S.P. Graphene quantum dots as a green photosensitizer with carbon-doped ZnO nanorods for quantum-dot-sensitized solar cell applications. Bull. Mater. Sci. 2019, 42, 65.
- Formo, E.; Lee, E.; Campbell, D.; Xia, Y. Functionalization of Electrospun TiO2 Nanofibers with Pt Nanoparticles and Nanowires for Catalytic Applications. Nano Lett. 2008, 8, 668–672.
- Yu, Y.; Xu, D. Single-crystalline TiO2 nanorods: Highly active and easily recycled photocatalysts. Appl. Catal. B 2007, 73, 166–171.
- Kumaresan, L.; Mahalakshmi, M.; Palanichamy, M.; Murugesan, V. Synthesis, Characterization, and Photocatalytic Activity of Sr2+ Doped TiO2 Nanoplates. Ind. Eng. Chem. Res. 2010, 49, 1480–1485.
- Zhang, K.; Zhou, W.; Chi, L.; Zhang, X.; Jiang, Z.J.C. Black N/H-TiO2 Nanoplates with a Flower-Like Hierarchical Architecture for Photocatalytic Hydrogen Evolution. ChemSusChem 2016, 9, 2841–2848.
- Tang, Y.; Wee, P.; Lai, Y.; Wang, X.; Gong, D.; Kanhere, P.; Lim, T.; Dong, Z.; Chen, Z. Hierarchical TiO2 Nanoflakes and Nanoparticles Hybrid Structure for Improved Photocatalytic Activity. J. Phys. Chem. C 2012, 116, 2772–2780.
- Sauvage, F.; Fonzo, F.D.; Bassi, A.L.; Casari, C.S.; Graetzel, M.J.N.L. Hierarchical TiO2 Photoanode for Dye-Sensitized Solar Cells. Nano Lett. 2010, 10, 2562–2567.
- Macak, J.M.; Albu, S.P.; Schmuki, P. Towards ideal hexagonal self-ordering of TiO2 nanotubes. Phys. Status Solidi RRL 2007, 1, 181–183.
- Fang, W.Q.; Zhou, J.Z.; Liu, J.; Chen, Z.G.; Yang, C.; Sun, C.H.; Qian, G.R.; Zou, J.; Qiao, S.Z.; Yang, H.G. Hierarchical Structures of Single-Crystalline Anatase TiO2 Nanosheets Dominated by {001} Facets. Chem. Eur. J. 2011, 17, 1423–1427.
- Li, H.; Bian, Z.; Zhu, J.; Zhang, D.; Li, G.; Huo, Y.; Li, H.; Lu, Y. Mesoporous Titania Spheres with Tunable Chamber Stucture and Enhanced Photocatalytic Activity. J. Am. Chem. Soc. 2007, 129, 8406–8407.
- Kondo, Y.; Yoshikawa, H.; Awaga, K.; Murayama, M.; Mori, T.; Sunada, K.; Bandow, S.; Iijima, S. Preparation, Photocatalytic Activities, and Dye-Sensitized Solar-Cell Performance of Submicron-Scale TiO2 Hollow Spheres. Langmuir 2008, 24, 547–550.
- Chen, S.; Yu, M.; Han, W.P.; Yan, X.; Liu, Y.C.; Zhang, J.C.; Zhang, H.D.; Yu, G.F.; Long, Y.Z. Electrospun anatase TiO2 nanorods for flexible optoelectronic devices. RSC Adv. 2014, 4, 46152–46156.
- Roy, P.; Berger, S.; Schmuki, P. TiO2 Nanotubes: Synthesis and Applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939.
- Macwan, D.P.; Dave, P.N.; Chaturvedi, S. A review on nano-TiO2 sol-gel type syntheses and its applications. J. Mater. Sci. 2011, 46, 3669–3686.
- Chen, S.; Liu, W.; Zhang, S.; Chen, Y. Preparation and activity evaluation of relative p-n junction photocatalyst Co-TiO2/TiO2. J. Sol-Gel Sci. Technol. 2010, 54, 258–267.