Coenzyme Q10 (CoQ10) is a compound with a crucial role in mitochondrial bioenergetics and membrane antioxidant protection. Despite the ubiquitous endogenous biosynthesis, specific medical conditions are associated with low circulating CoQ10 levels. However, previous studies of oral CoQ10 supplementation yielded inconsistent outcomes. In this text, previous CoQ10 trials are reviewed, either single or in combination with other nutrients, and stratified the study participants according to their metabolic statuses and medical conditions. The CoQ10 supplementation trials in elders reported many favorable outcomes. However, the single intervention was less promising when the host metabolic statuses were worsening with the likelihood of multiple nutrient insufficiencies, as in patients with an established diagnosis of metabolic or immune-related disorders. On the contrary, the mixed CoQ10 supplementation with other interacting nutrients created more promising impacts in hosts with compromised nutrient reserves. Furthermore, the results of either single or combined intervention will be less promising in far-advanced conditions with established damage, such as neurodegenerative disorders or cancers. With the limited high-level evidence studies on each host metabolic category, it could only be concluded that the considerations of whether to take supplementation varied by the individuals’ metabolic status and their nutrient reserves. Further studies are warranted.
- coenzyme Q10
- dietary supplements
- ubiquinone
- mitochondria
- bioenergetics
1. Introduction
2. Physiological Roles of CoQ10 in Humans
2.1. CoQ10 Roles in Mitochondrial Bioenergetics
2.2. CoQ10 Role as an Antioxidant
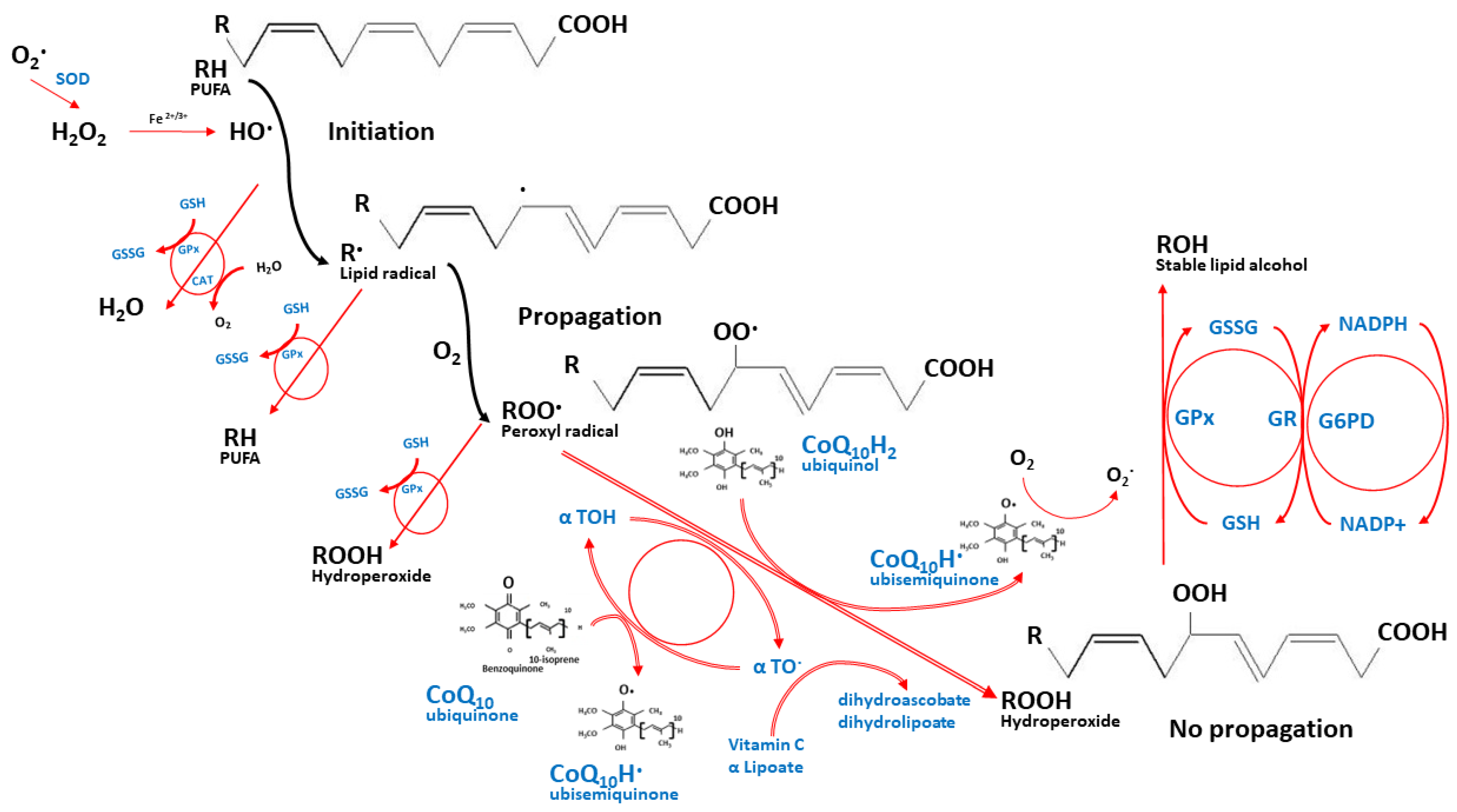
2.3. Other Physiological Roles of CoQ10
3. Potential Roles of CoQ10 Supplementation in Specific Medical Conditions
Despite the ubiquitous endogenous biosynthesis, specific medical conditions are associated with low circulating CoQ10 levels. However, the inconsistent results of CoQ10 interventions, from previous studies, implied the presence of unaccounted factors that contributed to clinical outcomes. After reviewing the participants’ status in previous CoQ10 clinical trials, two potentially confounding aspects are proposed herein, i.e., differences in host metabolic status and the need for CoQ10 interacting nutrients.
In conclusion, no single nutrient could magically drive whole physiological processes. Single CoQ10 supplementation will be beneficial only for hosts that specifically require it, such as hereditary CoQ10 deficiencies. The single intervention will be less promising when the host metabolic status worsens with the likelihood of multiple nutrient insufficiencies. On the contrary, the mixed CoQ10 supplementation with other interacting nutrients will create more promising impacts in hosts with compromised nutrient reserves. However, the results of either single or combined intervention will be less promising in far-advanced conditions with established damage.
With the limited amount of high-level evidence, such as provided by systematic reviews and meta-analyses, the researchers could only conclude that the considerations of whether to take supplementation varied by the individuals’ metabolic status and their nutrient reserves, which span across the continuum of metabolic triage processes that lead to chronic health issues. Future studies are warranted, particularly for the RCT with the design to control the host metabolic and nutrient status of participants and the meta-analysis of upcoming CoQ10 studies on each subject’s metabolic status.
This entry is adapted from the peer-reviewed paper 10.3390/nu14204383
References
- Crane, F.L.; Hatefi, Y.; Lester, R.L.; Widmer, C. Isolation of a quinone from beef heart mitochondria. Biochim. Biophys. Acta 1957, 25, 220–221.
- Garrido-Maraver, J.; Cordero, M.D.; Oropesa-Avila, M.; Fernandez Vega, A.; de la Mata, M.; Delgado Pavon, A.; de Miguel, M.; Perez Calero, C.; Villanueva Paz, M.; Cotan, D.; et al. Coenzyme q10 therapy. Mol Syndr. 2014, 5, 187–197.
- Nomenclature of Quinones with Isoprenoid Side-Chains. Eur. J. Biochem. 1975, 53, 15–18.
- Saini, R. Coenzyme Q10: The essential nutrient. J. Pharm. Bioallied Sci. 2011, 3, 466–467.
- Acosta, M.J.; Vazquez Fonseca, L.; Desbats, M.A.; Cerqua, C.; Zordan, R.; Trevisson, E.; Salviati, L. Coenzyme Q biosynthesis in health and disease. Biochim. Biophys. Acta 2016, 1857, 1079–1085.
- Clement, A.M. The Antioxidant Defense Network: Synergistic Combinations to Prevent Oxidative Damage; Brigham Young University: Provo, UT, USA, 2008.
- Trevisson, E.; DiMauro, S.; Navas, P.; Salviati, L. Coenzyme Q deficiency in muscle. Curr. Opin. Neurol. 2011, 24, 449–456.
- Quinzii, C.M.; Tadesse, S.; Naini, A.; Hirano, M. Effects of inhibiting CoQ10 biosynthesis with 4-nitrobenzoate in human fibroblasts. PLoS ONE 2012, 7, e30606.
- Laredj, L.N.; Licitra, F.; Puccio, H.M. The molecular genetics of coenzyme Q biosynthesis in health and disease. Biochimie 2014, 100, 78–87.
- Rodick, T.C.; Seibels, D.R.; Babu, J.R.; Huggins, K.W.; Ren, G.; Mathews, S.T. Potential role of coenzyme Q 10 in health and disease conditions. Nutr. Diet. Suppl. 2018, 10, 1–11.
- Palamakula, A.; Soliman, M.; Khan, M.M. Regional permeability of coenzyme Q10 in isolated rat gastrointestinal tracts. Pharmazie 2005, 60, 212–214.
- Bhagavan, H.N.; Chopra, R.K. Coenzyme Q10: Absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic. Res. 2006, 40, 445–453.
- Turunen, M.; Olsson, J.; Dallner, G. Metabolism and function of coenzyme Q. Biochim. Biophys. Acta 2004, 1660, 171–199.
- Cirilli, I.; Damiani, E.; Dludla, P.V.; Hargreaves, I.; Marcheggiani, F.; Millichap, L.E.; Orlando, P.; Silvestri, S.; Tiano, L. Role of Coenzyme Q10 in Health and Disease: An Update on the Last 10 Years (2010–2020). Antioxidants 2021, 10, 1325.
- Overvad, K.; Diamant, B.; Holm, L.; Holmer, G.; Mortensen, S.A.; Stender, S. Coenzyme Q10 in health and disease. Eur. J. Clin. Nutr. 1999, 53, 764–770.
- Orlando, P.; Silvestri, S.; Galeazzi, R.; Antonicelli, R.; Marcheggiani, F.; Cirilli, I.; Bacchetti, T.; Tiano, L. Effect of ubiquinol supplementation on biochemical and oxidative stress indexes after intense exercise in young athletes. Redox Rep. Commun. Free Radic. Res. 2018, 23, 136–145.
- Kalen, A.; Appelkvist, E.L.; Dallner, G. Age-related changes in the lipid compositions of rat and human tissues. Lipids 1989, 24, 579–584.
- Ates, O.; Bilen, H.; Keles, S.; Alp, H.H.; Keleş, M.S.; Yıldırım, K.; Ondaş, O.; Pınar, L.C.; Civelekler, M.; Baykal, O. Plasma coenzyme Q10 levels in type 2 diabetic patients with retinopathy. Int. J. Ophthalmol. 2013, 6, 675–679.
- Suksomboon, N.; Poolsup, N.; Juanak, N. Effects of coenzyme Q10 supplementation on metabolic profile in diabetes: A systematic review and meta-analysis. J. Clin. Pharm. Ther. 2015, 40, 413–418.
- Nawarskas, J.J. HMG-CoA reductase inhibitors and coenzyme Q10. Cardiol. Rev. 2005, 13, 76–79.
- Littarru, G.P.; Langsjoen, P. Coenzyme Q10 and statins: Biochemical and clinical implications. Mitochondrion 2007, 7, S168–S174.
- Tomasetti, M.; Alleva, R.; Borghi, B.; Collins, A.R. In vivo supplementation with coenzyme Q10 enhances the recovery of human lymphocytes from oxidative DNA damage. FASEB J. 2001, 15, 1425–1427.
- García-García, F.J.; Monistrol-Mula, A.; Cardellach, F.; Garrabou, G. Nutrition, Bioenergetics, and Metabolic Syndrome. Nutrients 2020, 12, 2785.
- Navas, P.; Villalba, J.M.; de Cabo, R. The importance of plasma membrane coenzyme Q in aging and stress responses. Mitochondrion 2007, 7, S34–S40.
- Zhang, Y.; Liu, J.; Chen, X.-Q.; Oliver Chen, C.Y. Ubiquinol is superior to ubiquinone to enhance Coenzyme Q10 status in older men. Food Funct. 2018, 9, 5653–5659.
- Lopez-Lluch, G.; Del Pozo-Cruz, J.; Sanchez-Cuesta, A.; Cortes-Rodriguez, A.B.; Navas, P. Bioavailability of coenzyme Q10 supplements depends on carrier lipids and solubilization. Nutrition 2019, 57, 133–140.
- Delkhosh, A.; Shoorei, H.; Niazi, V.; Delashoub, M.; Gharamaleki, M.N.; Ahani-Nahayati, M.; Dehaghi, Y.K.; Raza, S.; Taheri, M.H.; Mohaqiq, M.; et al. Coenzyme Q10 ameliorates inflammation, oxidative stress, and testicular histopathology in rats exposed to heat stress. Hum. Exp. Toxicol. 2021, 40, 3–15.
- Akbari, A.; Mobini, G.R.; Agah, S.; Morvaridzadeh, M.; Omidi, A.; Potter, E.; Fazelian, S.; Ardehali, S.H.; Daneshzad, E.; Dehghani, S. Coenzyme Q10 supplementation and oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Eur. J. Clin. Pharm. 2020, 76, 1483–1499.
- Thomas, S.R.; Stocker, R. Mechanisms of antioxidant action of ubiquinol-10 for low-density lipoprotein. In COENZYME Q; Kagan, V.E., Quinn, P.J., Eds.; CRC Press: Boca Raton, FL, USA, 2001; p. 131.
- Uekaji, Y.; Nakata, D.; Shiga, H.; Jo, A.; Tachi, I.; Fukumi, H.; Urano, A.; Terao, K. Formation of CoQ10 reduced form by mixing CoQ10 oxidized form γCD complex and vitamin C in powder. J. Incl. Phenom. Macrocycl. Chem. 2011, 70, 447–451.
- Zaki, N.M. Strategies for oral delivery and mitochondrial targeting of CoQ10. Drug Deliv. 2016, 23, 1868–1881.
- Ernster, L.; Dallner, G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim. Et Biophys. Acta 1995, 1271, 195–204.
- Pacanowski, M.A.; Frye, R.F.; Enogieru, O.; Schofield, R.S.; Zineh, I. Plasma Coenzyme Q10 Predicts Lipid-lowering Response to High-Dose Atorvastatin. J. Clin. Lipidol. 2008, 2, 289–297.
- Kagan, V.E.; Quinn, P.J. Coenzyme Q: Molecular Mechanisms in Health and Disease; CRC Press: Boca Raton, FL, USA, 2000.
- Genova, M.L.; Lenaz, G. Functional role of mitochondrial respiratory supercomplexes. Biochim. Biophys. Acta 2014, 1837, 427–443.
- Guo, R.; Zong, S.; Wu, M.; Gu, J.; Yang, M. Architecture of Human Mitochondrial Respiratory Megacomplex I2III2IV2. Cell 2017, 170, 1247–1257.e12.
- Milenkovic, D.; Blaza, J.N.; Larsson, N.G.; Hirst, J. The Enigma of the Respiratory Chain Supercomplex. Cell Metab. 2017, 25, 765–776.
- Hernandez-Camacho, J.D.; Bernier, M.; Lopez-Lluch, G.; Navas, P. Coenzyme Q10 Supplementation in Aging and Disease. Front. Physiol. 2018, 9, 44.
- Choo, H.J.; Kholmukhamedov, A.; Zhou, C.; Jobe, S. Inner Mitochondrial Membrane Disruption Links Apoptotic and Agonist-Initiated Phosphatidylserine Externalization in Platelets. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1503–1512.
- Schenkel, L.C.; Bakovic, M. Formation and regulation of mitochondrial membranes. Int. J. Cell Biol. 2014, 2014, 709828.
- Crane, F.L. Biochemical functions of coenzyme Q10. J. Am. Coll. Nutr. 2001, 20, 591–598.
- Alcazar-Fabra, M.; Navas, P.; Brea-Calvo, G. Coenzyme Q biosynthesis and its role in the respiratory chain structure. Biochim. Biophys. Acta 2016, 1857, 1073–1078.
- Schmelzer, C.; Lindner, I.; Rimbach, G.; Niklowitz, P.; Menke, T.; Doring, F. Functions of coenzyme Q10 in inflammation and gene expression. BioFactors 2008, 32, 179–183.
- Linnane, A.W.; Kopsidas, G.; Zhang, C.; Yarovaya, N.; Kovalenko, S.; Papakostopoulos, P.; Eastwood, H.; Graves, S.; Richardson, M. Cellular redox activity of coenzyme Q10: Effect of CoQ10 supplementation on human skeletal muscle. Free Radic. Res. 2002, 36, 445–453.
- Zhai, J.; Bo, Y.; Lu, Y.; Liu, C.; Zhang, L. Effects of Coenzyme Q10 on Markers of Inflammation: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0170172.
- Fan, L.; Feng, Y.; Chen, G.C.; Qin, L.Q.; Fu, C.L.; Chen, L.H. Effects of coenzyme Q10 supplementation on inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. Off. J. Ital. Pharmacol. Soc. 2017, 119, 128–136.
- Mazidi, M.; Kengne, A.P.; Banach, M. Effects of coenzyme Q10 supplementation on plasma C-reactive protein concentrations: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. Off. J. Ital. Pharmacol. Soc. 2018, 128, 130–136.
- Farsi, F.; Heshmati, J.; Keshtkar, A.; Irandoost, P.; Alamdari, N.M.; Akbari, A.; Janani, L.; Morshedzadeh, N.; Vafa, M. Can coenzyme Q10 supplementation effectively reduce human tumor necrosis factor-α and interleukin-6 levels in chronic inflammatory diseases? A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. Off. J. Ital. Pharmacol. Soc. 2019, 148, 104290.
- Ames, B.N. Musings in the twilight of my career. Free Radic. Biol. Med. 2022, 178, 219–225.
- Ames, B.N. Optimal micronutrients delay mitochondrial decay and age-associated diseases. Mech. Ageing Dev. 2010, 131, 473–479.
- McCann, J.C.; Ames, B.N. Vitamin K, an example of triage theory: Is micronutrient inadequacy linked to diseases of aging? Am. J. Clin. Nutr. 2009, 90, 889–907.
- Ames, B.N. Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. Proc. Natl. Acad. Sci. USA 2006, 103, 17589–17594.
