Etch pits could form on an exposed surface of a crystal when the crystal is exposed to an etching environment or chemicals. Due to different dissolution rates along various crystalline directions in a crystal, the dissolution process is anisotropic; hence, etch pits usually have a regular shape. The morphology, size, and density of etch pits can be affected by various factors, including the chemical composition of the etchant, etching time, etching temperature, status of the matrix, and so on. Traditionally, etch pits are utilized to evaluate the dislocation density and some defect-related properties. Now, in the modern fabrication industries, the relationship between etch pits and defects has been utilized more skillfully. High-quality crystals can be fabricated by controlling dislocations revealed by etch pits. Meanwhile, with the as-revealed dislocation as the diffusion path of atoms, new crystals will emerge in corresponding etch pits.
- etch pit
- crystal
- dislocation
- orientation
- defects
1. Introduction
2. Influencing Factors for the Formation of Etch Pits
2.1. The Etchant
2.1.1. Effect on the Morphology of Etch Pits
2.1.2. Effect on the Density of Etch Pits
2.1.3. Effect on the Etching Rate
2.2. The Etching Time
2.2.1. Effect on the Morphology of Etch Pits
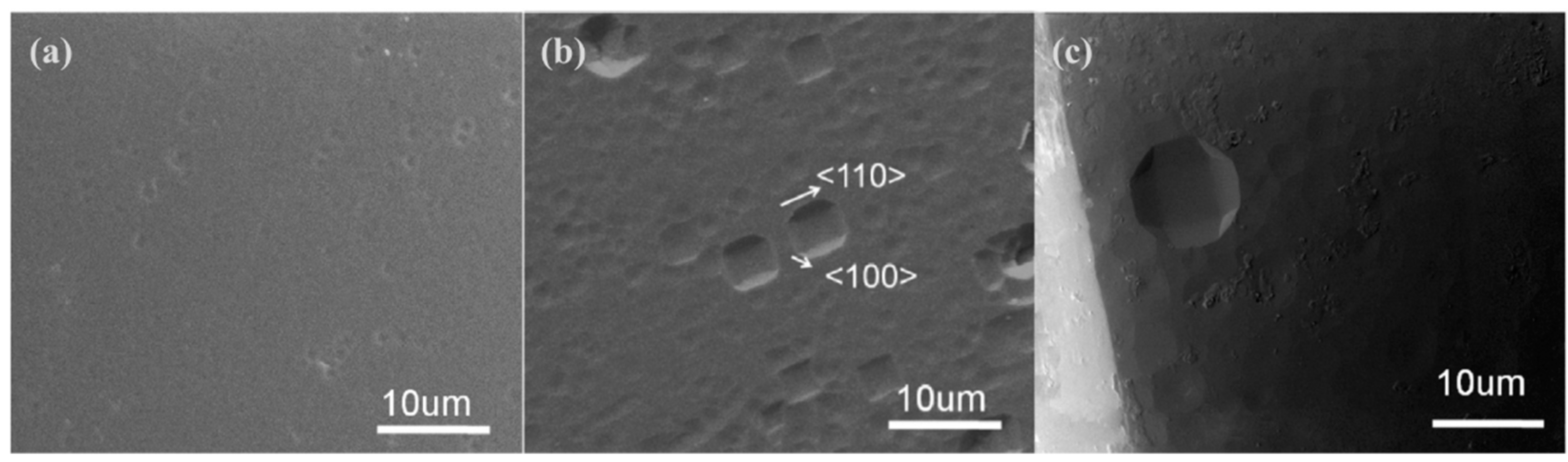
2.2.2. Effect on the Density of Etch Pits
2.3. The Etching Temperature
2.4. The Matrix
2.5. Electrochemical Parameters
2.6. The Atmosphere
2.7. Other Factors
3. Applications of Etch Pit Technology
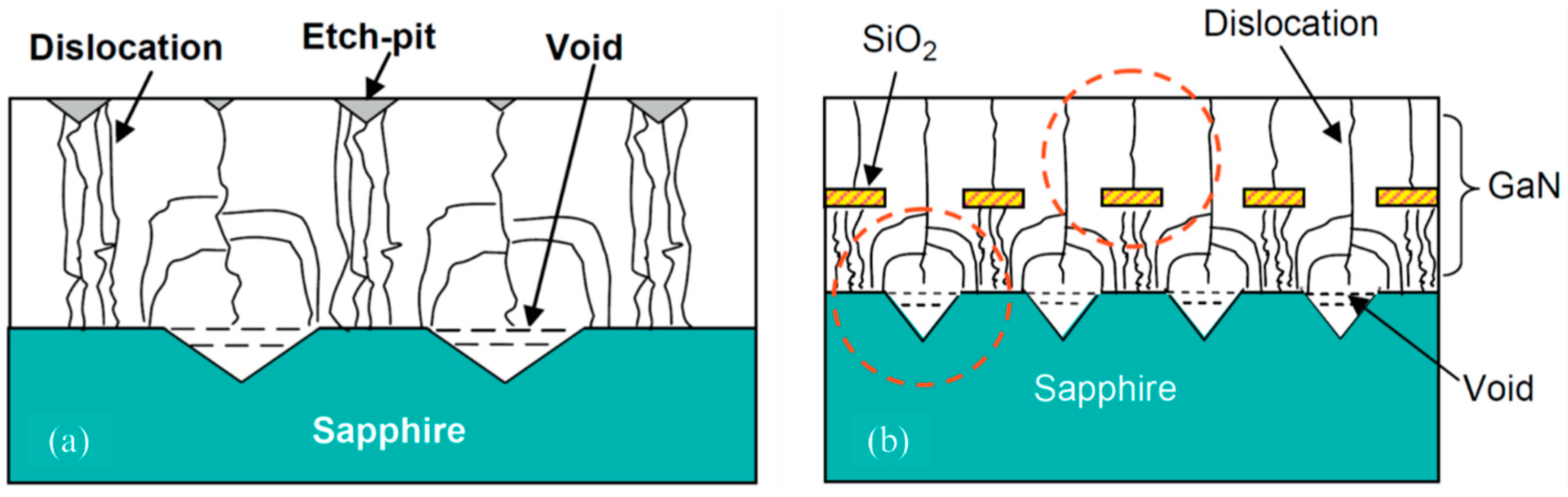
3.1. Investigation of Dislocations
3.2. Reduction of Dislocations
3.3. Count of Precipitates
3.4. Polytype Identification
3.5. Polarity Detemination
3.6. Detection of Leakage Current
4. Trends Related to Etch Pit Technology
4.1. Characterization of Etch Pits
4.2. Simulation of Disolution
4.3. Etching on Macro Patterns
4.4. Self-Assemble of Particles at Etch Pits
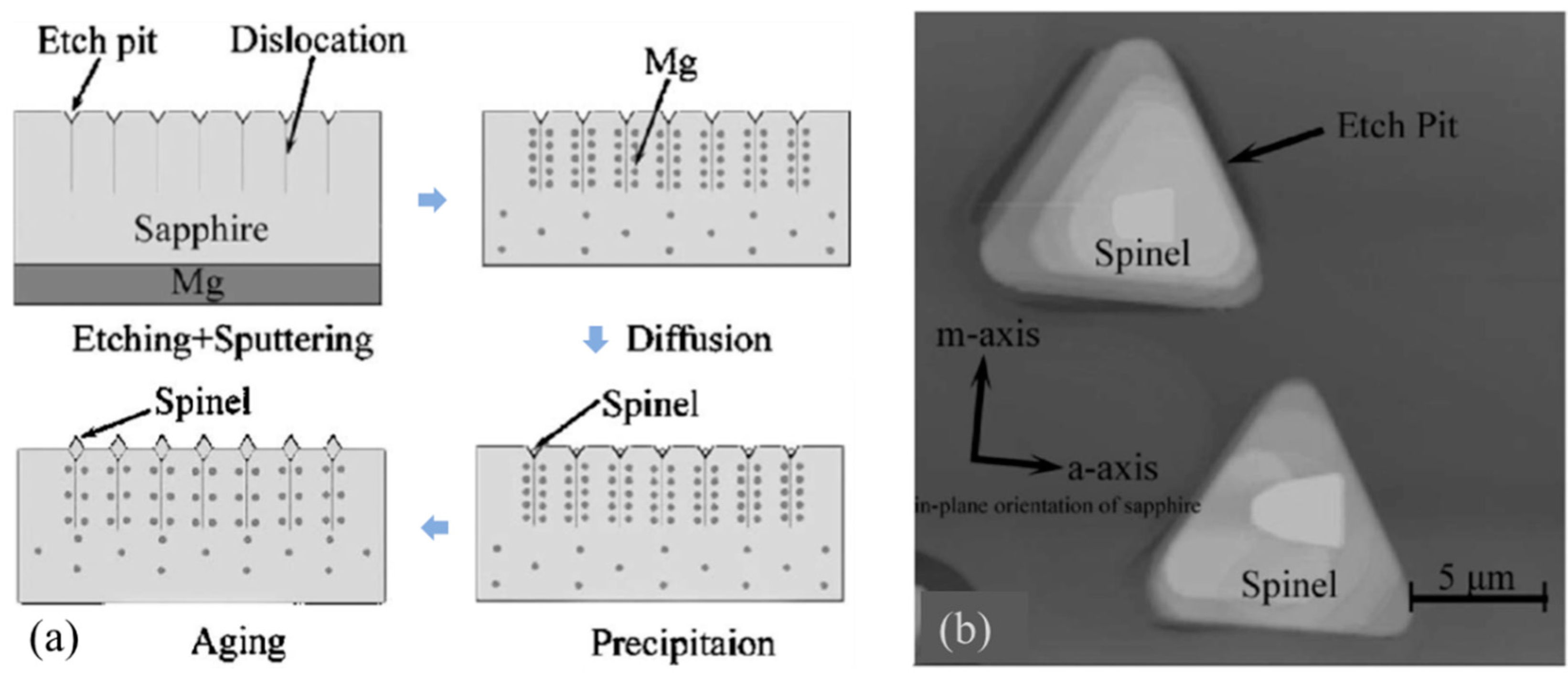
4.5. Crystal Growth at Etch Pits
This entry is adapted from the peer-reviewed paper 10.3390/cryst12111549
References
- Gautier, J.M.; Oelkers, E.H.; Schott, J. Are quartz dissolution rates proportional to B.E.T. surface areas? Geochim. Cosmochim. Acta 2001, 65, 1059–1070.
- Pina, C.M.; Pimentel, C.; García-Merino, M. High resolution imaging of the dolomite (104) cleavage surface by atomic force microscopy. Surf. Sci. 2010, 604, 1877–1881.
- Mercier, M.; Raimi, M.K.; Bonpunt, L. Observation by transmission electron microscopy of etch figures obtained on an organic molecular crystal. Microsc. Res. Tech. 1992, 21, 53–58.
- Saito, K.; Sugawara, S.; Sato, T.; Guo, J.Q.; Tsai, A.P. Unique shapes of micro-pits formed in an Al-Pd-Mn icosahedral quasicrystal by anodic etching. Mater. Trans. JIM 2000, 41, 1221–1225.
- Lu, D.Z.; Wu, M.J. Observation of etch pits in Fe-36wt%Ni Invar alloy. Int. J. Miner. Metall. Mater. 2014, 21, 682–686.
- Bondokov, R.T.; Mueller, S.G.; Morgan, K.E.; Slack, G.A.; Schujman, S.; Wood, M.C.; Smart, J.A.; Schowalter, L.J. Large-area AlN substrates for electronic applications: An industrial perspective. J. Cryst. Growth 2008, 310, 4020–4026.
- He, Z.; Zhao, B.; Zhu, S.; Chen, B.; Huang, W.; Lin, L.; Feng, B. Crystal growth and dislocation etch pits observation of chalcopyrite CdSiP2. J. Cryst. Growth 2018, 481, 29–34.
- Jo, W.; Kim, S.J.; Kim, D.Y. Analysis of the etching behavior of ZnO ceramics. Acta Mater. 2005, 53, 4185–4188.
- Chen, J.; Wang, J.F.; Wang, H.; Zhu, J.J.; Zhang, S.M.; Zhao, D.G.; Jiang, D.S.; Yang, H.; Jahn, U.; Ploog, K.H. Measurement of threading dislocation densities in GaN by wet chemical etching. Semicond. Sci. Technol. 2006, 21, 1229–1235.
- Chaudhuri, J.; Lee, R.G.; Nyakiti, L.; Armstrong, J.; Gu, Z.; Edgar, J.H.; Wen, J.G. Transmission electron microscopy study of defect-selective etched (010) ScN crystals. Mater. Lett. 2008, 62, 27–29.
- Zeng, D.; Jie, W.; Wang, T.; Zhang, J.; Zha, G. Type and formation mechanism of thermal etch pit on annealed (111) CdZnTe surface. Thin Solid Films 2009, 517, 2896–2899.
- Vaghayenegar, M.; Jacobs, R.N.; Benson, J.D.; Stoltz, A.J.; Almeida, L.A.; Smith, D.J. Correlation of Etch Pits and Dislocations in As-grown and Thermal Cycle-Annealed HgCdTe(211) Films. J. Electron. Mater. 2017, 46, 5007–5019.
- Adkins, J.F.; Naviaux, J.D.; Subhas, A.V.; Dong, S.; Berelson, W.M. The Dissolution Rate of CaCObinf3einf in the Ocean. Annu. Rev. Mar. Sci. 2021, 13, 57–80.
- Hino, T.; Tomiya, S.; Miyajima, T.; Yanashima, K.; Hashimoto, S.; Ikeda, M. Characterization of threading dislocations in GaN epitaxial layers. Appl. Phys. Lett. 2000, 76, 3421–3423.
- Xu, L.; Yu, B.; Yu, G.; Liu, H.; Zhang, L.; Li, X.; Huang, P.; Wang, B.; Wang, S. Study on the morphology of dislocation-related etch pits on pyramidal faces of KDP crystals. CrystEngComm 2021, 23, 2556–2562.
- Yang, J.R.; Cao, X.L.; Wei, Y.F.; He, L. Traces of HgCdTe defects as revealed by etch pits. J. Electron. Mater. 2008, 37, 1241–1246.
- Lantreibecq, A.; Legros, M.; Plassat, N.; Monchoux, J.P.; Pihan, E. Spatial distribution of structural defects in Cz-seeded directionally solidified silicon ingots: An etch pit study. J. Cryst. Growth 2018, 483, 183–189.
- Corke, N.T.; Kawada, A.A.; Sherwood, J.N. Etching of Dislocations in Crystals of Aromatic Hydrocarbons. Nature 1967, 213, 62–63.
- Sato, K.; Okada, M. Etching on large single crystals of stearic acid. Nature 1977, 269, 399–400.
- Mukerji, S.; Kar, T. Etch pit study of different crystallographic faces of L-arginine hydrobromide monohydrate (LAHBr) in some organic acids. J. Cryst. Growth 1999, 204, 341–347.
- Hashimoto, T.; Wu, F.; Speck, J.S.; Nakamura, S. A GaN bulk crystal with improved structural quality grown by the ammonothermal method. Nat. Mater. 2007, 6, 568–571.
- Kawamura, F.; Tanpo, M.; Miyoshi, N.; Imade, M.; Yoshimura, M.; Mori, Y.; Kitaoka, Y.; Sasaki, T. Growth of GaN single crystals with extremely low dislocation density by two-step dislocation reduction. J. Cryst. Growth 2009, 311, 3019–3024.
- Benson, J.D.; Smith, P.J.; Jacobs, R.N.; Markunas, J.K.; Jaime-Vasquez, M.; Almeida, L.A.; Stoltz, A.; Bubulac, L.O.; Groenert, M.; Wijewarnasuriya, P.S.; et al. Topography and dislocations in (112)B HgCdTe/CdTe/Si. J. Electron. Mater. 2009, 38, 1771–1775.
- Lu, L.; Gao, Z.Y.; Shen, B.; Xu, F.J.; Huang, S.; Miao, Z.L.; Hao, Y.; Yang, Z.J.; Zhang, G.Y.; Zhang, X.P.; et al. Microstructure and origin of dislocation etch pits in GaN epilayers grown by metal organic chemical vapor deposition. J. Appl. Phys. 2008, 104, 123525.
- Sadrabadi, P.; Durst, K.; Goken, M.; Blum, W. Quantification of dislocation structures at high resolution by atomic force microscopy of dislocation etch pits. Philos. Mag. Lett. 2009, 89, 391–398.
- Hatayama, T.; Shimizu, T.; Kouketsu, H.; Yano, H.; Uraoka, Y.; Fuyuki, T. Thermal etching of 4H-SiC(0001) Si faces in the mixed gas of chlorine and oxygen. Jpn. J. Appl. Phys. 2009, 48, 066516.
- Kallinger, B.; Polster, S.; Berwian, P.; Friedrich, J.; Mller, G.; Danilewsky, A.N.; Wehrhahn, A.; Weber, A.D. Threading dislocations in n- and p-type 4HSiC material analyzed by etching and synchrotron X-ray topography. J. Cryst. Growth 2011, 314, 21–29.
- Habel, F.; Seyboth, M. Determination of dislocation density in epitaxially grown GaN using an HCl etching process. Phys. Status Solidi C Conf. 2003, 2451, 2448–2451.
- Shah, I.A.; van der Wolf, B.M.A.; van Enckevort, W.J.P.; Vlieg, E. Wet chemical etching of silicon : Etch pit analysis by the Lichtfigur method. J. Cryst. Growth 2009, 311, 1371–1377.
- Shah, I.A.; van der Wolf, B.M.A.; van Enckevort, W.J.P.; Vlieg, E. Wet Chemical Etching of Silicon : Autocatalysis in Pit Formation. J. Electrochem. Soc. 2008, 155, J79.
- Bickermann, M.; Schmidt, S.; Epelbaum, B.M.; Heimann, P.; Nagata, S.; Winnacker, A. Wet KOH etching of freestanding AlN single crystals. J. Cryst. Growth 2007, 300, 299–307.
- Fleck, M.; Zuschlag, A.; Hahn, G. Etch Pit Density Reduction in POCl3 and Atmospheric Pressure Chemical Vapor Deposition-Gettered mc-Si. Phys. Status Solidi Appl. Mater. Sci. 2019, 216, 1900316.
- Persichetti, L.; Fanfoni, M.; De Seta, M.; Di Gaspare, L.; Ottaviano, L.; Goletti, C.; Sgarlata, A. Formation of extended thermal etch pits on annealed Ge wafers. Appl. Surf. Sci. 2018, 462, 86–94.
- Velbel, M.A. Dissolution of olivine during natural weathering. Geochim. Cosmochim. Acta 2009, 73, 6098–6113.
- Bhandari, J.; Khan, F.; Abbassi, R.; Garaniya, V.; Ojeda, R. Modelling of pitting corrosion in marine and offshore steel structures—A technical review. J. Loss Prev. Process Ind. 2015, 37, 39–62.
- Xu, W.; Zhang, B.; Deng, Y.; Wang, Z.; Jiang, Q.; Yang, L.; Zhang, J. Corrosion of rail tracks and their protection. Corros. Rev. 2021, 39, 1–13.
- Frankel, G.S. Pitting Corrosion of Metals. Corrosion 1998, 145, 2186–2198.
- Campbell, J. The Mechanisms of Metallurgical Failure The origin of Fracture; Matthew Deans: Oxford, UK, 2020.
- Soltis, J. Passivity breakdown, pit initiation and propagation of pits in metallic materials—Review. Corros. Sci. 2015, 90, 5–22.
- Adamczyk, K.; Stokkan, G.; Di Sabatino, M. Guidelines for establishing an etching procedure for dislocation density measurements on multicrystalline silicon samples. MethodsX 2018, 5, 1178–1186.
- Lu, M.; Chang, X.; Li, Z.L.; Yang, Z.J.; Zhang, G.Y.; Zhang, B. Etch pits and threading dislocations in GaN films grown by metal-organic chemical vapour deposition. Chin. Phys. Lett. 2003, 20, 398–400.
- Britt, D.W.; Hlady, V. In-situ atomic force microscope imaging of calcite etch pit morphology changes in undersaturated and 1-hydroxyethylidene-1,1-diphosphonic acid poisoned solutions. Langmuir 1997, 13, 1873–1876.
- Na, K.H.; Pyun, S. Il Effects of SO42−, S2O32− and HSO4− Ion additives on the pitting corrosion of pure aluminium in 1 M NaCl solution at 40–70 °C. J. Solid State Electrochem. 2005, 9, 639–645.
- Yao, Y.-Z.; Ishikawa, Y.; Sugawara, Y.; Saitoh, H.; Danno, K.; Suzuki, H.; Kawai, Y.; Shibata, N. Molten KOH Etching with Na2O2 Additive for Dislocation Revelation in 4H-SiC Epilayers and Substrates. Jpn. J. Appl. Phys. 2011, 50, 075502.
- Lin, C.S.; Li, W.J. Pitting Behavior of Aluminum Foil during Alternating Current Etching in Hydrochloric Acid Containing Sulfate Ions. J. Electrochem. Soc. 2006, 153, C51.
- Hsu, H.C.; Su, Y.K.; Cheng, S.H.; Huang, S.J.; Cao, J.M.; Chen, K.C. Investigation of etch characteristics of non-polar GaN by wet chemical etching. Appl. Surf. Sci. 2010, 257, 1080–1083.
- Shen, G.; Zhao, Y.; Sun, J.; Liu, J.; Xie, H.; Yang, J.; Dong, Z. HCl-H2SO4-H2O solution etching behavior of InAs (1 0 0) surface. J. Cryst. Growth 2020, 547, 125800.
- Jianrong, Y.; Huiming, G.; Xinqiang, C.; Weizheng, F.; Li, H. Dislocation assessment of CdZnTe by chemical etching on both (1 1 1)B and (2 1 1)B faces. J. Cryst. Growth 2002, 234, 337–342.
- Zhang, L.; Shao, Y.; Wu, Y.; Hao, X.; Chen, X.; Qu, S.; Xu, X. Characterization of dislocation etch pits in HVPE-grown GaN using different wet chemical etching methods. J. Alloys Compd. 2010, 504, 186–191.
- Min, L.; Xin, C.; Hui-Zhi, F.; Zhi-Jian, Y.; Hua, Y.; Zi-Lan, L.; Qian, R.; Guo-Yi, Z.; Bei, Z. Etch-pits and threading dislocations in thick LEO GaN films on sapphire grown by MOCVD. Phys. Status Solidi C Conf. 2004, 1, 2438–2440.
- Karan, S.; Sen Gupta, S.; Sen Gupta, S.P. Revelation of dislocation etch pits in mixed crystals of ammonium-potassium sulphate, 2SO4. J. Cryst. Growth 2001, 233, 555–560.
- Han, S.-C.; Kim, J.-K.; Kim, J.Y.; Kim, K.-K.; Tampo, H.; Niki, S.; Lee, J.-M. Formation of Hexagonal Pyramids and Pits on V-/VI-Polar and III-/II-Polar GaN/ZnO Surfaces by Wet Etching. J. Electrochem. Soc. 2010, 157, D60.
- Tiwari, R.N.; Chang, L. Etching of GaN by microwave plasma of hydrogen. Semicond. Sci. Technol. 2010, 25, 035010.
- Wang, K.S.; Resch, R.; Dunn, K.; Shuler, P.; Tang, Y.; Koel, B.E.; Yen, T.F. Scanning force microscopy study of etch pits formed during dissolution of a barite (001) surface in CDTA and EDTA solutions. Langmuir 2000, 16, 649–655.
- Karuppasamy, P.; Kamalesh, T.; Anitha, K.; Abdul Kalam, S.; Senthil Pandian, M.; Ramasamy, P.; Verma, S.; Venugopal Rao, S. Synthesis, crystal growth, structure and characterization of a novel third order nonlinear optical organic single crystal: 2-Amino 4,6-Dimethyl Pyrimidine 4-nitrophenol. Opt. Mater. 2018, 84, 475–489.
- Hanada, K.; Moribayashi, T.; Koshi, K.; Sasaki, K.; Kuramata, A.; Ueda, O.; Kasu, M. Origins of etch pits in β-Ga2O3(010) single crystals. Jpn. J. Appl. Phys. 2016, 55, 1202BG.
- Osawa, N.; Fukuoka, K. Pit nucleation behavior of aluminium foil for electrolytic capacitors during early stage of DC etching. Corros. Sci. 2000, 42, 585–597.
- Khokhryakov, A.F.; Palyanov, Y.N. Revealing of dislocations in diamond crystals by the selective etching method. J. Cryst. Growth 2006, 293, 469–474.
- Ono, S.; Habazaki, H. Pit growth behaviour of aluminium under galvanostatic control. Corros. Sci. 2011, 53, 3521–3525.
- Li, L.; Chen, X.; Zhang, W.; Peng, K. Characterization and formation mechanism of pits on diamond face etched by molten potassium nitrite. Int. J. Refract. Met. Hard Mater. 2018, 71, 129–134.
- Cui, Y.; Hu, X.; Xie, X.; Xu, X. Threading dislocation classification for 4H-SiC substrates using the KOH etching method. CrystEngComm 2018, 20, 978–982.
- Yasuda, N.; Koguchi, Y.; Tsubomatsu, M.; Takagi, T.; Kobayashi, I.; Tsuruta, T.; Morishima, H. Extremely high dose neutron dosimetry using CR-39 and atomic force microscopy. Radiat. Prot. Dosim. 2006, 120, 470–474.
- Kachroo, S.K.; Bamzai, K.K.; Dhar, P.R.; Kotru, P.N.; Wanklyn, B.M. Etch patterns on flux grown DyFeO3 crystal surfaces. Mater. Chem. Phys. 2001, 68, 72–76.
- Gu, Z.; Edgar, J.H.; Coffey, D.W.; Chaudhuri, J.; Nyakiti, L.; Lee, R.G.; Wen, J.G. Defect-selective etching of scandium nitride crystals. J. Cryst. Growth 2006, 293, 242–246.
- Petukhov, V.; Bakin, A.; El-Shaer, A.H.; Mofor, A.C.; Waag, A. Etch-pit density investigation on both polar faces of ZnO substrates. Electrochem. Solid-State Lett. 2007, 10, H357.
- Habuka, H.; Fukumoto, Y.; Kato, T. Off-Orientation Influence on C-Face (0001) 4H-SiC Surface Morphology Produced by Etching Using Chlorine Trifluoride Gas. ECS J. Solid State Sci. Technol. 2013, 2, N3025–N3027.
- Robey, S.W.; Sinniah, K. Initial etching of GaAs (001) during plasma cleaning. J. Appl. Phys. 2000, 88, 2994–2998.
- Wu, P. Etching study of dislocations in heavily nitrogen doped SiC crystals. J. Cryst. Growth 2010, 312, 1193–1198.
- Khokhryakov, A.F.; Palyanov, Y.N. Effect of nitrogen impurity on etching of synthetic diamond crystals. J. Cryst. Growth 2015, 430, 71–74.
- Lee, J.; Kim, J.; Kim, J.; Lee, J.; Chung, H.; Tak, Y. Effects of pretreatment on the aluminium etch pit formation. Corros. Sci. 2009, 51, 1501–1505.
- Farrell, S.; Rao, M.V.; Brill, G.; Chen, Y.; Wijewarnasuriya, P.; Dhar, N.; Benson, J.D.; Harris, K. Comparison of the schaake and benson etches to delineate dislocations in HgCdTe layers. J. Electron. Mater. 2013, 42, 3097–3102.
- Sundararajan, S.P.; Crouse, D.; Lo, Y.-H. Gallium nitride: Method of defect characterization by wet oxidation in an oxalic acid electrolytic cell. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. 2002, 20, 1339.
- Ono, S.; Habazaki, H. Role of cathodic half-cycle on AC etch process of aluminium. Corros. Sci. 2010, 52, 2164–2171.
- Stallcup, R.E.; Mo, Y.; Scharf, T.W.; Perez, J.M. Formation of nanometer-size high-density pits on epitaxial diamond (100) films. Diam. Relat. Mater. 2007, 16, 1727–1731.
- Wheeler, E.K.; Whitman, P.K.; Land, T.A.; De Yoreo, J.; Thorsness, C.B.; McWhirter, J.H.; Hanna, M.L.; Miller, E.L. Investigation of etch pits on KDP crystals with porous sol-gel coatings. Appl. Phys. A Mater. Sci. Process. 2002, 74, 813–823.
- Ohashi, T.; Sugimoto, W.; Takasu, Y. Catalytic etching of -oriented diamond coating with Fe, Co, Ni, and Pt nanoparticles under hydrogen. Diam. Relat. Mater. 2011, 20, 1165–1170.
- Ivashchenko, V.E.; Boldyrev, V.V.; Zakharov, Y.A.; Shakhtshneider, T.P.; Ermakov, A.E.; Krasheninin, V.I. The effect of magnetic field on the shape of etch pits of paracetamol crystals. Mater. Res. Innov. 2002, 5, 214–218.
- Weyher, J.L. Characterization of Compound Semiconductors by Etching. In Concise Encyclopedia of Semiconducting Materials & Related Technologies; Pergamon: Oxford, UK, 1992; pp. 37–44.
- McDougall, D.J. Etch pits. In Mineralogy; Frye, K., Ed.; Springer: Boston, MA, USA, 1983; pp. 150–152.
- Cao, Y.; Ni, S.; Liao, X.; Song, M.; Zhu, Y. Structural evolutions of metallic materials processed by severe plastic deformation. Mater. Sci. Eng. R Rep. 2018, 133, 1–59.
- Gale, W.F.; Totemeier, T.C. (Eds.) Smithells Metals Reference Book; Elsevier: Oxford, UK, 2004; p. 26.
- Nicolov, M. Shaped single crystals of CaF2. J. Cryst. Growth 2000, 218, 62–66.
- Javaid, F.; Bruder, E.; Durst, K. Indentation size effect and dislocation structure evolution in (001) oriented SrTiO3 Berkovich indentations: HR-EBSD and etch-pit analysis. Acta Mater. 2017, 139, 1–10.
- Hossain, A.; Bolotnikov, A.E.; Camarda, G.S.; Cui, Y.; Yang, G.; James, R.B. Defects in cadmium zinc telluride crystals revealed by etch-pit distributions. J. Cryst. Growth 2008, 310, 4493–4498.
- Yao, Y.; Sugawara, Y.; Ishikawa, Y.; Okada, N.; Tadatomo, K. Crystallinity Evaluation and Dislocation Observation for an Aluminum Nitride Single-Crystal Substrate on a Wafer Scale. J. Electron. Mater. 2020, 49, 5144–5153.
- Cui, X.P.; Fang, W.Z.; Sun, S.W.; Zhang, C.J.; Xu, H.L.; Yang, J.R. Characteristics of the dislocations in CdZnTe crystals revealed by etch pits. J. Cryst. Growth 2011, 321, 40–44.
- Lisovenko, V.A.; Khutornaya, L.A.; Shpak, M.T.; Velikaya, E.N. Basal dislocations in anthracene crystals. Phys. Status Solidi 1977, 42, 433–437.
- Motoki, K.; Okahisa, T.; Nakahata, S.; Matsumoto, N.; Kimura, H.; Kasai, H.; Takemoto, K.; Uematsu, K.; Ueno, M.; Kumagai, Y.; et al. Preparation of large GaN substrates. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2002, 93, 123–130.
- Wuu, D.S.; Wu, H.W.; Chen, S.T.; Tsai, T.Y.; Zheng, X.; Horng, R.H. Defect reduction of laterally regrown GaN on GaN/patterned sapphire substrates. J. Cryst. Growth 2009, 311, 3063–3066.
- Hu, W.; Die, J.; Wang, C.; Yan, S.; Hu, X.; Du, C.; Jiang, Y.; Deng, Z.; Wang, L.; Jia, H.; et al. The substantial dislocation reduction by preferentially passivating etched defect pits in GaN epitaxial growth. Appl. Phys. Express 2019, 12, 035502.
- Lee, M.; Mikulik, D.; Yang, M.; Park, S. Nearly perfect GaN crystal via pit-assisted growth by HVPE. CrystEngComm 2017, 19, 2036–2041.
- Leonhardt, D.; Han, S.M. New Method to Produce High-Quality Epitaxial Ge on Si Using SiO2-Lined Etch Pits and Epitaxial Lateral Overgrowth for III-V Integration. ECS Trans. 2012, 45, 147–149.
- Sheng, F.F.; Cui, X.P.; Sun, S.W.; Yang, J.R. Etch pits of precipitates in CdZnTe crystals on (1 1 1) B surface. J. Cryst. Growth 2012, 354, 76–80.
- Yang, Y.; Chen, Z. Identification of SiC polytypes by etched Si-face morphology. Mater. Sci. Semicond. Process. 2009, 12, 113–117.
- Muto, D.; Araki, T.; Naoi, H.; Matsuda, F.; Nanishi, Y. Polarity determination of InN by wet etching. Phys. Status Solidi Appl. Mater. Sci. 2005, 202, 773–776.
- Kasu, M.; Hanada, K.; Moribayashi, T.; Hashiguchi, A.; Oshima, T.; Oishi, T.; Koshi, K.; Sasaki, K.; Kuramata, A.; Ueda, O. Relationship between crystal defects and leakage current in β-Ga2O3 Schottky barrier diodes. Jpn. J. Appl. Phys. 2016, 55, 1202BB.
- Lee, S.W.; Oh, D.C.; Goto, H.; Ha, J.S.; Lee, H.J.; Hanada, T.; Cho, M.W.; Yao, T.; Hong, S.K.; Lee, H.Y.; et al. Origin of forward leakage current in GaN-based light-emitting devices. Appl. Phys. Lett. 2006, 89, 132117.
- Hahn, G.; Fleck, M. Automatic etch pit density analysis in multicrystalline silicon. Comput. Mater. Sci. 2020, 183, 109886.
- Palodhi, K.; Chatterjee, J.; Bhattacharyya, R.; Dey, S.; Ghosh, S.K.; Maulik, A.; Raha, S. Convolution based hybrid image processing technique for microscopic images of etch-pits in Nuclear Track Detectors. Radiat. Meas. 2020, 130, 106219.
- Wzorek, M.; Czerwinski, A.; Ratajczak, J.; Dylewicz, R.; Katcki, J. Selective etching of dislocations in GaN and quantitative SEM analysis with shape-reconstruction method. Micron 2009, 40, 37–40.
- Sorai, M.; Ohsumi, T.; Ishikawa, M.; Tsukamoto, K. Feldspar dissolution rates measured using phase-shift interferometry: Implications to CO2 underground sequestration. Appl. Geochem. 2007, 22, 2795–2809.
- Van Driessche, A.E.S.; García-Ruíz, J.M.; Tsukamoto, K.; Patiño-Lopez, L.D.; Satoh, H. Ultraslow growth rates of giant gypsum crystals. Proc. Natl. Acad. Sci. USA 2011, 108, 15721–15726.
- Sato, F.; Kuchimaru, T.; Kato, Y.; Iida, T. Digital image analysis of etch pit formation in CR-39 track detector. Jpn. J. Appl. Phys. 2008, 47, 269–272.
- Kurganskaya, I.; Luttge, A. A comprehensive stochastic model of phyllosilicate dissolution: Structure and kinematics of etch pits formed on muscovite basal face. Geochim. Cosmochim. Acta 2013, 120, 545–560.
- Stübner, K.; Jonckheere, R.; Ratschbacher, L. Revelation of nuclear tracks and dislocations: A Monte Carlo simulation of mineral etching. Geochim. Cosmochim. Acta 2008, 72, 3184–3199.
- Ono, S.; Uchibori, K.; Asoh, H. Control of nano/microstructure and pit initiation sites on aluminium surface by use of self-assembled spheres. Surf. Interface Anal. 2010, 42, 264–268.
- Brevnov, D.A. Electrochemical etching of patterned Al (100) foils in HCl. J. Micromech. Microeng. 2008, 18, 2–7.
- Chen, H.M.; Suen, Y.W.; Chen, S.J.; Luo, G.L.; Lai, Y.P.; Chen, S.T.; Lee, C.H.; Kuan, C.H. Effect of surface Si redistribution on the alignment of Ge dots grown on pit-patterned Si(001) substrates. Nanotechnology 2014, 25, 475301.
- Liu, C.M.; Chen, J.C. Growth of Mg-Al spinel microcrystals on a sapphire surface using a solution-precipitation method. Appl. Phys. Lett. 2006, 89, 7–10.
