Neural tube defects (NTDs) are a group of birth defects in which an opening in the spine or cranium remains from early in human development. In the third week of pregnancy called gastrulation, specialized cells on the dorsal side of the embryo begin to change shape and form the neural tube. When the neural tube does not close completely, an NTD develops. Specific types include: spina bifida which affects the spine, anencephaly which results in little to no brain, encephalocele which affects the skull, and iniencephaly which results in severe neck problems. NTDs are one of the most common birth defects, affecting over 300,000 births each year worldwide. For example, spina bifida affects approximately 1,500 births annually in the United States, or about 3.5 in every 10,000 (0.035% of US births), which has decreased from around 5 per 10,000 (0.05% of US births) since folate fortification of grain products was started. The number of deaths in the US each year due to neural tube defects also declined from 1,200 before folate fortification was started to 840.
- neural tube defects
- encephalocele
- spina bifida
1. Types
There are two classes of NTDs: open, which are more common, and closed. Open NTDs occur when the brain and/or spinal cord are exposed at birth through a defect in the skull or vertebrae (spinal column). Open NTDs include anencephaly, encephaloceles, hydranencephaly, iniencephaly, schizencephaly, and the most common form, spina bifida. Closed NTDs occur when the spinal defect is covered by skin. Types of closed NTDs include lipomeningocele, lipomyelomeningocele, and tethered cord.
1.1. Anencephaly
Anencephaly (without brain) is a severe neural tube defect that occurs when the anterior-most end of the neural tube fails to close, usually during the 23rd and 26th days of pregnancy. This results in an absence of a major portion of the brain and skull. Infants born with this condition lack the main part of the forebrain and are usually blind, deaf and display major craniofacial anomalies. The lack of a functioning cerebrum will prevent the infant from even gaining consciousness. Infants are either stillborn or usually die within a few hours or days after birth. For example, anencephaly in humans can result from mutations in the NUAK2 kinase.[1]
1.2. Encephaloceles
Encephaloceles are characterized by protrusions of the brain through the skull that are sac-like and covered with membrane. They can be a groove down the middle of the upper part of the skull, between the forehead and nose, or the back of the skull. Due to the range in its location, encephaloceles are classified by the location as well as the type of defect it causes. Subtypes include occipital encephalocele, encephalocele of the carnival vault, and nasal encephaloceles (frontoethmoidal encephaloceles and basal encephaloceles), with approximately 80% of all encephaloceles occurring in the occipital area.[2] Encephaloceles are often obvious and diagnosed immediately. Sometimes small encephaloceles in the nasal and forehead are undetected.[3] Despite the wide range in its implications, encephaloceles are most likely to be caused by improper separation of the surface ectoderm and the neuroectoderm after the closure of the neural folds in the fourth week of gastrulation.[4]
1.3. Hydranencephaly
Hydranencephaly is a condition in which the cerebral hemispheres are missing and instead filled with sacs of cerebrospinal fluid. People are born with hydranencephaly, but most of the time, the symptoms appear in a later stage. Newborns with hydrancephaly can swallow, cry, sleep and their head is in proportion to their body. However, after a few weeks, the infants develop increased muscle tone and irritability. After a few months, the brain start to fill with cerebrospinal fluid (hydrocephalus). This has several consequences. Infants start to develop problems with seeing, hearing, growing, and learning. The missing parts of the brain and the amount of cerebrospinal fluid can also lead to seizures, spasm, problems with regulating their body temperature, and breathing and digestion problems. Besides problems in the brain, hydranencephaly can also be seen on the outside of the body. Hydrocephalus leads to more cerebrospinal fluid in the brain, which can result in an enlarged head.[5][6][7]
The cause of hydranencephaly is not clear. Hydranencephaly is a result of an injury of the nervous system or an abnormal development of the nervous system. The neural tube closes in the sixth week of the pregnancy,[8] so hydranencephaly develops during these weeks of the pregnancy. The cause of these injuries/development is not clear.
Theories regarding the causes of hydrancephaly include:[7]
- blockage in the carotid artery: some researchers think that a blockage of the carotid artery leads to the under-/no development of the brain. The carotid artery is the most important blood supplier of the brain. With a blockage, the brain barely receives blood. Blood is necessary for development and keeping intact of the brain.[7]
- inherited condition.[7]
- infections: during the pregnancy, a woman can develop an infection in the uterus what can lead to problems with the neural tube.[7]
- environmental toxins: during the pregnancy, a woman can be exposed to environmental toxins what can have effect on the health of the infant.[7]
1.4. Iniencephaly
Iniencephaly is a rare neural tube defect that results in extreme bending of the head to the spine. The diagnosis can usually be made on antenatal ultrasound scanning, but if not will undoubtedly be made immediately after birth because the head is bent backwards and the face looks upwards. Usually the neck is absent. The skin of the face connects directly to the chest and the scalp connects to the upper back. Individuals with iniencephaly generally die within a few hours after birth.
1.5. Spina Bifida
Spina bifida is further divided into two subclasses, spina bifida cystica and spina bifida occulta.
- Spina bifida cystica includes meningocele and myelomeningocele. Meningocele is less severe and is characterized by herniation of the meninges, but not the spinal cord, through the opening in the spinal canal. Myelomeningocele involves herniation of the meninges as well as the spinal cord through the opening.[9]
- Spina bifida occulta means hidden split spine.[10] In this type of neural tube defect, the meninges do not herniate through the opening in the spinal canal.[9] The most frequently seen form of spina bifida occulta is when parts of the bones of the spine, called the spinous process, and the neural arch appear abnormal on a radiogram, without involvement of the spinal cord and spinal nerves.[11] The risk of recurrence in those who have a first degree relative (a parent or sibling) is 5–10 times greater compared to the general population.
2. Causes
2.1. Folate Deficiency
Inadequate levels of folate (vitamin B9) and vitamin B12 during pregnancy have been found to lead to increased risk of NTDs.[12][13] Although both are part of the same biopathway, folate deficiency is much more common and therefore more of a concern.[12][13] Folate is required for the production and maintenance of new cells, for DNA synthesis and RNA synthesis. Folate is needed to carry one carbon groups for methylation and nucleic acid synthesis. It has been hypothesized that the early human embryo may be particularly vulnerable to folate deficiency due to differences of the functional enzymes in this pathway during embryogenesis combined with high demand for post translational methylations of the cytoskeleton in neural cells during neural tube closure.[14] Failure of post-translational methylation of the cytoskeleton, required for differentiation has been implicated in neural tube defects.[15] Vitamin B12 is also an important receptor in the folate biopathway such that studies have shown deficiency in vitamin B12 contributes to risk of NTDs as well.[16] There is substantial evidence that direct folic supplementation increases blood serum levels of bioavailable folate even though at least one study have shown slow and variable activity of dihydrofolate reductase in human liver.[17][18] A diet rich in natural folate (350 μg/d) can show as much increase in plasma folate as taking low levels of folic acid (250 μg/d) in individuals[19] However a comparison of general population outcomes across many countries with different approaches to increasing folate consumption has found that only general food fortification with folic acid reduces neural tube defects.[20] While there have been concerns about folic acid supplementation being linked to an increased risk for cancer, a systematic review in 2012 shows there is no evidence except in the case of prostate cancer which indicates a modest reduction in risk.[21]
There have been studies showing the relationship between NTDs, folate deficiency and the difference of skin pigmentation within human populations across different latitudes. There are many factors that would influence the folate levels in human bodies: (i) the direct dietary intake of folic acid through fortified products, (ii) environmental agents such as UV radiation. In concern with the latter, the UV radiation-induced folate photolysis has been shown via in vitro and in vivo studies to decrease the folate level and implicate in etiology of NTDs not only in humans but other amphibian species. Therefore, a protection against the UV radiation-induced photolysis of folate is imperative for the evolution of human populations living in tropical regions where the exposure to UV radiation is high over the year. One body natural adaptation is to elevate the concentration of melanin inside the skin. Melanin works as either an optical filter to disperse the incoming UV radiation rays or free radical to stabilize the hazardous photochemical products. Multiple studies have demonstrated the highly melanized integument as a defense against folate photolysis in Native Americans or African Americans correlates with lower occurrence of NTDs in general.[22][23]
2.2. Genetic Deficiencies
As reported by Bruno Reversade and colleagues, the inactivation of the NUAK2 kinase in humans leads to anencephaly.[1] This fatal birth defect is believed to arise as a consequence of impaired HIPPO signalling.[1] Other genes such as TRIM36 have also been associated with anencephaly in humans.[24]
2.3. Gene-Environment Interaction
A deficiency of folate itself does not cause neural tube defects. The association seen between reduced neural tube defects and folic acid supplementation is due to a gene-environment interaction such as vulnerability caused by the C677T methylenetetrahydrofolate reductase (MTHFR) variant. Supplementing folic acid during pregnancy reduces the prevalence of NTDs by not exposing this otherwise sub-clinical mutation to aggravating conditions.[25] Other potential causes can include folate antimetabolites (such as methotrexate), mycotoxins in contaminated corn meal, arsenic, hyperthermia in early development, and radiation.[26][27][28] Maternal obesity has also been found to be a risk factor for NTDs.[29] Studies have shown that both maternal cigarette smoking and maternal exposure to secondhand smoke increased the risk for neural tube defects in offspring.[30] A mechanism by which maternal exposure to cigarette smoke could increase NTD risk in offspring is suggested by several studies that show an association between cigarette smoking and elevations of homocysteine levels. Cigarette smoke during pregnancy, including secondhand exposure, can increase the risk of neural tube defects.[31] All of the above may act by interference with some aspect of normal folic acid metabolism and folate linked methylation related cellular processes as there are multiple genes of this type associated with neural tube defects.[32]
2.4. Other
Folic acid supplementation reduces the prevalence of neural tube defects by approximately 70% of neural tube defects indicating that 30% are not folate-dependent and are due to some cause other than alterations of methylation patterns.[33] Multiple other genes related to neural tube defects exist which are candidates for folate insensitive neural tube defects.[32] There are also several syndromes such as Meckel syndrome, and triploid syndrome which are frequently accompanied by neural tube defects that are assumed to be unrelated to folate metabolism[34]
3. Diagnosis
Tests for neural tube defects include ultrasound examination and measurement of maternal serum alpha-fetoprotein (MSAFP). Second trimester ultrasound is recommended as the primary screening tool for NTDs, and MSAFP as a secondary screening tool.[35] This is due to increased safety, increased sensitivity and decreased false positive rate of ultrasound as compared to MSAFP.[35] Amniotic fluid alpha-fetoprotein (AFAFP) and amniotic fluid acetylcholinesterase (AFAChE) tests are also used to confirming if ultrasound screening indicates a positive risk.[36] Often, these defects are apparent at birth, but acute defects may not be diagnosed until much later in life. An elevated MSAFP measured at 16–18 weeks gestation is a good predictor of open neural tube defects, however the test has a very high false positive rate, (2% of all women tested in Ontario, Canada between 1993 and 2000 tested positive without having an open neural tube defect, although 5% is the commonly quoted result worldwide) and only a portion of neural tube defects are detected by this screen test (73% in the same Ontario study).[37] MSAFP screening combined with routine ultrasonography has the best detection rate although detection by ultrasonography is dependent on operator training and the quality of the equipment.[38][39]
4. Prevention
Incidence of neural tube defects has been shown to decline through maintenance of adequate folic acid levels prior to and during pregnancy. This is achieved through dietary sources and supplementation of folic acid.[40] In 1996, the United States Food and Drug Administration published regulations requiring the addition of folic acid to enriched breads, cereals, flour and other grain products.[41] Similar regulations made it mandatory to fortify selected grain products with folic acid in Canada by 1998.[42] It is important to note that during the first four weeks of pregnancy (when most people do not even realize that they are pregnant), adequate folate intake is essential for proper operation of the neurulation process. Therefore, any individuals who could become pregnant are advised to eat foods fortified with folic acid or take supplements in addition to eating folate-rich foods to reduce the risks of serious birth defects.[43][44][45] In Canada, mandatory fortification of selected foods with folic acid had been shown to reduce the incidence of neural tube defects by 46% compared to incidence prior to mandatory fortification.[46] However, relying on eating a folate-rich diet alone is not recommended for preventing neural tube defects when trying to conceive because a regular diet usually does not contain enough folate to reach pregnancy requirements.[47][48] All individuals who have the ability to become pregnant are advised to get 400 micrograms of folic acid daily.[49][50] This daily 400 mcg dose of folic acid can be found in most multivitamins advertised as for women.[51] Higher doses can be found in pre-natal multivitamins but those doses may not be necessary for everyone.[52][53] Individuals who have previously given birth to a child with a neural tube defect and are trying to conceive again may benefit from a supplement containing 4.0 mg daily, following advice provided by their doctor.[51] In Canada, guidelines on folic acid intake when trying to conceive is based on a risk assessment of how likely they are to experience a neural tube defect during pregnancy. Risk is divided into high, moderate, and low risk categories.[50] High risk would include those that had a past experience with neural tube defects, either themselves or during another pregnancy.[50] Medium risk individuals are those with certain conditions that put them at higher risk for experiencing a neural tube defect. These include having a first or second degree relative or partner with a history of neural tube defects, having a gastrointestinal condition that affects normal absorption patterns, advanced kidney disease, kidney dialysis, alcohol over-use, or had another pregnancy resulting in a congenital abnormality that was folate sensitive. Medium risk individuals would also include those taking medications that can interfere with folate absorption such as anticonvulsants, metformin, sulfasalazine, triamterene, and trimethoprim.[50] Low risk would include everyone else that do not fall into either medium or high risk categories. Recommendations on when to start folic acid supplementation for all individuals looking to become pregnant is at least 3 months preconception.[48][50] If an individual is in the high risk category, the recommended dose is 4–5 mg of folic acid daily until 12 weeks gestation and then decrease to 0.4–1 mg until 4–6 weeks postpartum or for however long breastfeeding lasts.[50] If an individual is in the medium risk category, the recommended dose is 1 mg of folic acid daily until 12 weeks gestation and then they can either continue at 1 mg or decrease to 0.4 mg daily until 4–6 weeks postpartum or however long breastfeeding lasts.[50] If the pregnancy is low risk to develop a neural tube defect then the recommendation for that individual is 0.4 mg daily until 4–6 weeks postpartum or however long breastfeeding lasts.[50] All dose recommendations and risk assessment should be done with the advice of a qualified health care provider.[49]
5. Treatment
As of 2008, treatments of NTDs depends on the severity of the complication. No treatment is available for anencephaly and infants usually do not survive more than a few hours. Aggressive surgical management has improved survival and the functions of infants with spina bifida, meningoceles and mild myelomeningoceles. The success of surgery often depends on the amount of brain tissue involved in the encephalocele. The goal of treatment for NTDs is to allow the individual to achieve the highest level of function and independence. Fetal surgery in utero before 26 weeks gestation has been performed with some hope that there is benefit to the outcome including a reduction in Arnold–Chiari malformation and thereby decreases the need for a ventriculoperitoneal shunt but the procedure is very high risk for both mother and baby and is considered extremely invasive with questions that the positive outcomes may be due to ascertainment bias and not true benefit. Further, this surgery is not a cure for all problems associated with a neural tube defect. Other areas of research include tissue engineering and stem cell therapy but this research has not been used in humans.[54]
6. Epidemiology
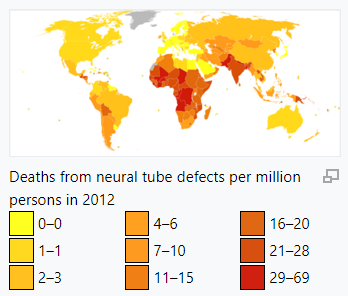
Neural tube defects resulted in 71,000 deaths globally in 2010.[55] It is unclear how common the condition is in low income countries.[56]
Prevalence rates of NTDs at birth used to be a reliable measure for the actual number of children affected by the diseases.[57] However, due to advances in technology and the ability to diagnose prenatally, the rates at birth are no longer reliable.[57] Measuring the number of cases at birth may be the most practical way, but the most accurate way would be to include stillbirths and live-births.[57] Most studies that calculate prevalence rates only include data from live births and stillborn children and normally exclude the data from abortions and miscarriages.[57] Abortions are a huge contributing factor to the prevalence rates; one study found that in 1986 only a quarter of the pregnancies with an identified NTD were aborted, but that number had already doubled by 1999.[57] Through this data, it is clear that excluding data from abortions could greatly affect the prevalence rates. This could also possibly explain why prevalence rates have appeared to drop. If abortions are not being included in the data but half of the identified cases are being aborted, the data could show that prevalence rates are dropping when they actually are not. However, it is unclear how much of an impact these could have on prevalence rates due to the fact that abortion rates and advances in technology vary greatly by country.[57]
There are many maternal factors that also play a role in prevalence rates of NTDs.[57] These factors include things like maternal age and obesity all the way to things like socioeconomic status along with many others.[57] Maternal age has not been shown to have a huge impact on prevalence rates, but when there has been a relationship identified, older mothers along with very young mothers are at an increased risk.[57] While maternal age may not have a huge impact, mothers that have a body mass index greater than 29 double the risk of their child having an NTD.[57] Studies have also shown that mothers with three or more previous children show moderate risk for their next child having an NTD.[57]
The content is sourced from: https://handwiki.org/wiki/Medicine:Neural_tube_defect
References
- Bonnard, Carine; Navaratnam, Naveenan; Ghosh, Kakaly; Chan, Puck Wee; Tan, Thong Teck; Pomp, Oz; Ng, Alvin Yu Jin; Tohari, Sumanty et al. (2020-12-07). "A loss-of-function NUAK2 mutation in humans causes anencephaly due to impaired Hippo-YAP signaling". The Journal of Experimental Medicine 217 (12). doi:10.1084/jem.20191561. ISSN 1540-9538. PMID 32845958. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=7953732
- Abdel-Aziz, Mosaad; El-Bosraty, Hussam (2010). ""Nasal encephalocele: Endoscopic excision with anesthetic consideration"". International Journal of Pediatric Otorhinolaryngology 74 (8): 869–873. doi:10.1016/j.ijporl.2010.04.015. PMID 20554034. https://www.sciencedirect.com/science/article/pii/S0165587610002065#!. Retrieved 2020-11-19.
- "Encephaloceles Information Page". National institute of Neurological Disorders and Stroke. https://www.ninds.nih.gov/Disorders/All-Disorders/Encephaloceles-Information-Page.
- Broekman, Marike; Hoving, Eelco (2008). ""Nasal encephalocele in a child with Beckwith-Wiedemann syndrome"". Journal of Neurosurgery 6 (1): 485–7. doi:10.3171/PED/2008/1/6/485. PMID 18518702. https://pubmed.ncbi.nlm.nih.gov/18518702/. Retrieved 2020-11-19.
- "Hydranencephaly Information Page". 2019-03-27. https://www.ninds.nih.gov/disorders/all-disorders/hydranencephaly-information-page.
- "Hydranencephaly" (in en-US). https://rarediseases.org/rare-diseases/hydranencephaly/.
- "Hydranencephaly: Symptoms, Causes, Complications, and Treatment" (in en). 24 July 2017. https://www.healthline.com/health/hydranencephaly.
- "Fetal development: What happens during the first trimester?" (in en). https://www.mayoclinic.org/healthy-lifestyle/pregnancy-week-by-week/in-depth/prenatal-care/art-20045302.
- Le, Tao; Bhushan, Vikas; Vasan, Neil (2010). First Aid for the USMLE Step 1: 2010 (20th ed.). McGraw-Hill. p. 127. ISBN 978-0-07-163340-6. https://archive.org/details/firstaidforusmle0000unse_r6p4/page/127.
- Saladin, Kenneth (2010). Anatomy and Physiology: The Unity of Form and Function. McGraw-Hill. p. 485. ISBN 978-0-07-352569-3. https://archive.org/details/anatomyphysiolog00sala_868.
- Pittman, T (2008). "Spina bifida occulta.". Journal of Neurosurgery. Pediatrics 1 (2): 113. doi:10.3171/PED/2008/1/2/113. PMID 18352777. https://dx.doi.org/10.3171%2FPED%2F2008%2F1%2F2%2F113
- Molloy, A. M.; Kirke, P. N.; Troendle, J. F.; Burke, H.; Sutton, M.; Brody, L. C.; Scott, JM; Mills, JL (2009). "Maternal Vitamin B-12 Status and Risk of Neural Tube Defects in a Population With High Neural Tube Defect Prevalence and No Folic Acid Fortification". Pediatrics 123 (3): 917–23. doi:10.1542/peds.2008-1173. PMID 19255021. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=4161975
- De-Regil, Luz Maria; Peña-Rosas, Juan Pablo; Fernández-Gaxiola, Ana C.; Rayco-Solon, Pura (2015-12-14). "Effects and safety of periconceptional oral folate supplementation for preventing birth defects". The Cochrane Database of Systematic Reviews 2015 (12): CD007950. doi:10.1002/14651858.CD007950.pub3. ISSN 1469-493X. PMID 26662928. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=8783750
- Bjorklund N, Gordon R (2006). "A hypothesis linking low folate intake to neural tube defects due to failure of post-translation methylations of the cytoskeleton". Int. J. Dev. Biol. 50 (2–3): 135–41. doi:10.1387/ijdb.052102nb. PMID 16479482. http://www.ijdb.ehu.es/web/paper.php?doi=052102nb.
- Akchiche (2012). "Homocysteinylation of neuronal proteins contributes to folate deficiency-associated alterations of differentiation, vesicular transport, and plasticity in hippocampal neuronal cells". The FASEB Journal 26 (10): 3980–92. doi:10.1096/fj.12-205757. PMID 22713523. https://dx.doi.org/10.1096%2Ffj.12-205757
- Li, F.; Watkins, D.; Rosenblatt, D. S. (2009). "Vitamin B-12 and birth defects". Molecular Genetics and Metabolism 98 (1–2): 166–72. doi:10.1016/j.ymgme.2009.06.004. PMID 19586788. https://dx.doi.org/10.1016%2Fj.ymgme.2009.06.004
- Anderson C. (2013). "Response of serum and red blood cell folate concentrations to folic acid supplementation depends on methylenetetrahydrofolate reductase C677T genotype: results from a crossover trial". Mol. Nutr. Food Res. 57 (4): 637–44. doi:10.1002/mnfr.201200108. PMID 23456769. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=4132693
- "The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake". Proc Natl Acad Sci U S A 106 (36): 15424–29. Sep 2009. doi:10.1073/pnas.0902072106. PMID 19706381. Bibcode: 2009PNAS..10615424B. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2730961
- Brouwer, I. A.; Van Dusseldorp, M.; West, C. E.; Meyboom, S.; Thomas, C. M.; Duran, M.; Van Het Hof, K. H.; Eskes, T. K. et al. (1999). "Dietary Folate from Vegetables and Citrus Fruit Decreases Plasma Homocysteine Concentrations in Humans in a Dietary Controlled Trial". J. Nutr. 129 (6): 1135–39. doi:10.1093/jn/129.6.1135. PMID 10356077. https://dx.doi.org/10.1093%2Fjn%2F129.6.1135
- International retrospective cohort study of neural tube defects in relation to folic acid recommendations: are the recommendations working? BMJ 2005;330:571 http://www.bmj.com/content/330/7491/571?variant=full-text
- Wein, TN; Pike, E; Wisløff, T; Staff, A; Smeland, S; Klemp, M (12 January 2012). "Cancer risk with folic acid supplements: a systematic review and meta-analysis". BMJ Open 2 (1): e000653. doi:10.1136/bmjopen-2011-000653. PMID 22240654. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3278486
- Jablonski, Nina G.; Chaplin, George (2010-05-11). "Human skin pigmentation as an adaptation to UV radiation" (in en). Proceedings of the National Academy of Sciences 107 (Supplement 2): 8962–8968. doi:10.1073/pnas.0914628107. ISSN 0027-8424. PMID 20445093. Bibcode: 2010PNAS..107.8962J. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3024016
- "Evolution of Human Skin Coloration — Department of Anthropology". https://anth.la.psu.edu/research/research-labs/jablonski-lab/research/skin-evol.
- Singh, Nivedita; Kumble Bhat, Vishwanath; Tiwari, Ankana; Kodaganur, Srinivas G.; Tontanahal, Sagar J.; Sarda, Astha; Malini, K. V.; Kumar, Arun (2017-03-15). "A homozygous mutation in TRIM36 causes autosomal recessive anencephaly in an Indian family". Human Molecular Genetics 26 (6): 1104–1114. doi:10.1093/hmg/ddx020. ISSN 1460-2083. PMID 28087737. https://dx.doi.org/10.1093%2Fhmg%2Fddx020
- Yan, L; Zhao, L; Long, Y; Zou, P; Ji, G; Gu, A; Zhao, P (October 3, 2012). "Association of the Maternal MTHFR C677T Polymorphism with Susceptibility to Neural Tube Defects in Offsprings: Evidence from 25 Case-Control Studies". PLOS ONE 7 (10): e41689. doi:10.1371/journal.pone.0041689. PMID 23056169. Bibcode: 2012PLoSO...741689Y. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3463537
- Neural Tube Defects at eMedicine https://emedicine.medscape.com/article/1177162-overview
- Suarez, L.; Brender, J. D.; Langlois, P. H.; Zhan, F. B.; Moody, K. (2007). "Pregnant women taking medication for epilepsy have a higher chance of having a child with a neural tube defect. Maternal exposures to hazardous waste sites and industrial facilities and risk of neural tube defects in offspring". Annals of Epidemiology 17 (10): 772–77. doi:10.1016/j.annepidem.2007.05.005. PMID 17689262. https://dx.doi.org/10.1016%2Fj.annepidem.2007.05.005
- Zhou, F. C.; Fang, Y.; Goodlett, C. (2008). "Peptidergic Agonists of Activity-Dependent Neurotrophic Factor Protect Against Prenatal Alcohol-Induced Neural Tube Defects and Serotonin Neuron Loss". Alcoholism: Clinical and Experimental Research 32 (8): 1361–71. doi:10.1111/j.1530-0277.2008.00722.x. PMID 18565153. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2758042
- Huang, Hai-Yan; Chen, Hong-Lin; Feng, Li-Ping (March 2017). "Maternal obesity and the risk of neural tube defects in offspring: A meta-analysis". Obesity Research & Clinical Practice 11 (2): 188–197. doi:10.1016/j.orcp.2016.04.005. ISSN 1871-403X. PMID 27155922. https://dx.doi.org/10.1016%2Fj.orcp.2016.04.005
- Wang, M; Wang, ZP; Gong, R; Zhao, ZT (January 2014). "Maternal smoking during pregnancy and neural tube defects in offspring: a meta-analysis.". Child's Nervous System 30 (1): 83–89. doi:10.1007/s00381-013-2194-5. PMID 23760473. https://dx.doi.org/10.1007%2Fs00381-013-2194-5
- Meng, Xin; Sun, Yanxin; Duan, Wenhou; Jia, Chongqi (2018). "Meta-analysis of the association of maternal smoking and passive smoking during pregnancy with neural tube defects" (in en). International Journal of Gynecology & Obstetrics 140 (1): 18–25. doi:10.1002/ijgo.12334. ISSN 1879-3479. PMID 28963797. https://dx.doi.org/10.1002%2Fijgo.12334
- Greene ND, Stanier P, Copp AJ (October 2009). "Genetics of human neural tube defects". Hum. Mol. Genet. 18 (R2): R113–29. doi:10.1093/hmg/ddp347. PMID 19808787. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2758708
- "Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group". Lancet 338 (8760): 131–37. 1991. doi:10.1016/0140-6736(91)90133-a. PMID 1677062. https://dx.doi.org/10.1016%2F0140-6736%2891%2990133-a
- Rose, N, Mennuti, M, Glob. Fetal Neural Tube Defects: Diagnosis, Management, and Treatment libr. women's med., (ISSN 1756-2228) 2009; doi:10.3843/GLOWM.10224 http://www.glowm.com/section_view/heading/Fetal%20Neural%20Tube%20Defects:%20Diagnosis,%20Management,%20and%20Treatment/item/224
- Wilson, R. Douglas; Audibert, Francois; Brock, Jo-Ann; Campagnolo, Carla; Carroll, June; Cartier, Lola; Chitayat, David; Gagnon, Alain et al. (October 2014). "Prenatal Screening, Diagnosis, and Pregnancy Management of Fetal Neural Tube Defects". Journal of Obstetrics and Gynaecology Canada 36 (10): 927–939. doi:10.1016/s1701-2163(15)30444-8. PMID 25375307. https://dx.doi.org/10.1016%2Fs1701-2163%2815%2930444-8
- Milunsky, A; Alpert, E. (1984). "Results and benefits of a maternal serum alpha-fetoprotein screening program". JAMA 252 (11): 1438–42. doi:10.1001/jama.252.11.1438. PMID 6206249. https://dx.doi.org/10.1001%2Fjama.252.11.1438
- Summer Maternal Serum Screening in Ontario Using the Triple Marker Test J Med Screen September 2003 vol. 10 no. 3 107–11. http://msc.sagepub.com/content/10/3/107.short
- Boyd, PA; Devigan, C.; Khoshnood, B.; Loane, M.; Garne, E.; Dolk, H. (2008). "Survey of prenatal screening policies in Europe for structural malformations and chromosome anomalies, and their impact on detection and termination rates for neural tube defects and Down's syndrome". BJOG: An International Journal of Obstetrics and Gynaecology 115 (6): 689–696. doi:10.1111/j.1471-0528.2008.01700.x. PMID 18410651. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2344123
- Norem et.al Routine Ultrasonography Compared With Maternal Serum Alpha-fetoprotein for Neural Tube Defect Screening Obstetrics & Gynecology: October 2005 Vol 106:4 pp. 747–52 http://journals.lww.com/greenjournal/Abstract/2005/10000/Routine_Ultrasonography_Compared_With_Maternal.14.aspx
- De Wals, Philippe; Tairou, Fassiatou; Van Allen, Margot I.; Uh, Soo-Hong; Lowry, R. Brian; Sibbald, Barbara; Evans, Jane A.; Van den Hof, Michiel C. et al. (2007-07-12). "Reduction in Neural-Tube Defects after Folic Acid Fortification in Canada" (in en). New England Journal of Medicine 357 (2): 135–142. doi:10.1056/NEJMoa067103. ISSN 0028-4793. PMID 17625125. http://www.nejm.org/doi/abs/10.1056/NEJMoa067103.
- Daly, S; Mills, JL; Molloy, AM; Conley, M; Lee, YJ; Kirke, PN; Weir, DG; Scott, JM (1997). "Minimum effective dose of folic acid for food fortification to prevent neural-tube defects". Lancet 350 (9092): 1666–69. doi:10.1016/S0140-6736(97)07247-4. PMID 9400511. https://dx.doi.org/10.1016%2FS0140-6736%2897%2907247-4
- Ray, Joel G. (2004-06-01). "Folic Acid Food Fortification in Canada" (in en). Nutrition Reviews 62 (6): 35–39. doi:10.1301/nr.2004.jun.S35-S39. PMID 15298446. http://doi.wiley.com/10.1301/nr.2004.jun.S35-S39.
- Greene, ND; Stanier, P; Copp, AJ (2009). "Genetics of human neural tube defects". Human Molecular Genetics 18 (R2): R113–29. doi:10.1093/hmg/ddp347. PMID 19808787. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2758708
- Milunsky, A; Jick, H; Jick, SS; Bruell, CL; MacLaughlin, DS; Rothman, KJ; Willett, W (1989). "Multivitamin/folic acid supplementation in early pregnancy reduces the prevalence of neural tube defects". Journal of the American Medical Association 262 (20): 2847–52. doi:10.1001/jama.262.20.2847. PMID 2478730. https://dx.doi.org/10.1001%2Fjama.262.20.2847
- Goh, YI; Koren, G (2008). "Folic acid in pregnancy and fetal outcomes". J. Obstet. Gynaecol. 28 (1): 3–13. doi:10.1080/01443610701814195. PMID 18259891. https://dx.doi.org/10.1080%2F01443610701814195
- De Wals P; Tairou F; Van Allen MI et al. (2007). "Reduction in neural-tube defects after folic acid fortification in Canada". N Engl J Med 357 (2): 135–42. doi:10.1056/NEJMoa067103. PMID 17625125. https://dx.doi.org/10.1056%2FNEJMoa067103
- Naithani, Manisha; Saxena, Vartika; Mirza, Anissa Atif; Kumari, Ranjeeta; Sharma, Kapil; Bharadwaj, Jyoti (2016). "Assessment of Folic Acid Supplementation in Pregnant Women by Estimation of Serum Levels of Tetrahydrofolic Acid, Dihydrofolate Reductase, and Homocysteine" (in en). Scientifica 2016: 1–5. doi:10.1155/2016/1520685. ISSN 2090-908X. PMID 27064332. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=4811260
- Canada, Health (2009-04-28). "Prenatal Nutrition Guidelines for Health Professionals - Folate Contributes to a Healthy Pregnancy". https://www.canada.ca/en/health-canada/services/publications/food-nutrition/prenatal-nutrition-guidelines-health-professionals-folate-contributes-healthy-pregnancy-2009.html.
- Canada, Public Health Agency of (2018-01-05). "Folic acid and neural tube defects". https://www.canada.ca/en/public-health/services/pregnancy/folic-acid.html.
- Wilson, R. Douglas; Genetics Committee; Wilson, R. Douglas; Audibert, François; Brock, Jo-Ann; Carroll, June; Cartier, Lola; Gagnon, Alain et al. (June 2015). "Pre-conception Folic Acid and Multivitamin Supplementation for the Primary and Secondary Prevention of Neural Tube Defects and Other Folic Acid-Sensitive Congenital Anomalies". Journal of Obstetrics and Gynaecology Canada 37 (6): 534–552. doi:10.1016/s1701-2163(15)30230-9. ISSN 1701-2163. PMID 26334606. https://pubmed.ncbi.nlm.nih.gov/26334606.
- Centers for Disease Control (11 September 1992). "Recommendations for the Use of Folic Acid to Reduce the Number of Cases of Spina Bifida and Other Neural Tube Defects". Morbidity and Mortality Weekly Report 41 (RR-14): 001. https://www.cdc.gov/mmwr/preview/mmwrhtml/00019479.htm.
- CDC (2021-04-19). "Folic Acid" (in en-us). https://www.cdc.gov/ncbddd/folicacid/about.html.
- Canada, Health (2009-04-28). "Prenatal Nutrition Guidelines for Health Professionals - Folate Contributes to a Healthy Pregnancy". https://www.canada.ca/en/health-canada/services/publications/food-nutrition/prenatal-nutrition-guidelines-health-professionals-folate-contributes-healthy-pregnancy-2009.html.
- Sutton, Leslie N. (2008). "Fetal surgery for neural tube defects". Best Practice & Research Clinical Obstetrics & Gynaecology 22 (1): 175–188. doi:10.1016/j.bpobgyn.2007.07.004. PMID 17714997. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2293328
- Lozano, R (Dec 15, 2012). "Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010.". Lancet 380 (9859): 2095–128. doi:10.1016/S0140-6736(12)61728-0. PMID 23245604. https://zenodo.org/record/2557786.
- Zaganjor, Ibrahim; Sekkarie, Ahlia; Tsang, Becky L.; Williams, Jennifer; Razzaghi, Hilda; Mulinare, Joseph; Sniezek, Joseph E.; Cannon, Michael J. et al. (2016-04-11). "Describing the Prevalence of Neural Tube Defects Worldwide: A Systematic Literature Review". PLOS ONE 11 (4): e0151586. doi:10.1371/journal.pone.0151586. ISSN 1932-6203. PMID 27064786. Bibcode: 2016PLoSO..1151586Z. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=4827875
- Frey, Lauren; Hauser, W. Allen (2003). "Epidemiology of Neural Tube Defects" (in en). Epilepsia 44 (s3): 4–13. doi:10.1046/j.1528-1157.44.s3.2.x. ISSN 1528-1167. PMID 12790881. https://dx.doi.org/10.1046%2Fj.1528-1157.44.s3.2.x
