Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Polymer Science
As an excellent semiconductor photocatalyst, zinc oxide is widely used in the field of photocatalysis and is regarded as one of the most reliable materials to solve environmental problems. However, because its band gap energy limits the absorption of visible light and reduces the efficiency of catalytic degradation, it needs to be doped with other substances or compounded with other substances and precious metal.
- zinc oxide
- photocatalysis
- doping
1. Introduction
With the development of the times and the development of science and technology, such as textile, printing and dyeing, paint, medicine and other fields of industrial production will produce a large number of industrial wastewater discharged into the surrounding water, resulting in serious pollution [1,2,3]. The large number and unpredictable composition of organic dyes undoubtedly increase the difficulty of treatment. Traditional industrial wastewater treatment methods include flocculation-flocculation [4,5], electroflocculation [6], flocculation-carbon adsorption [7], electrochemistry, advanced oxidation, and biochemistry, etc.; however, these methods have some problems, such as high energy consumption, high cost, and unsatisfactory treatment effect. The semiconductor composites use light energy to create hole–electron pairs and use the electron holes in the surface active sites as reducing and oxidizing agents to promote redox, respectively. Therefore, the organic pollutants in sewage can be completely decomposed into inorganic small molecules, which has the characteristics of continuous and non-secondary pollution, and has been widely concerned. As a result, the inorganic semiconductor photocatalyst in recent years in the field of organic pollutants in the degradation of the important application prospects of attention [8,9,10,11].
Among the various photocatalysts, TiO2 that pass for the most widely employed “golden ”, photocatalyst has been largely used in heterogeneous photocatalysis, due to its chemical stability, nontoxicity [12,13,14]. Compared with titanium dioxide, zinc oxide has been found to have higher quantum efficiency and theoretical photocatalytic efficiency than titanium dioxide and it is a promising photocatalyst [15,16,17]. The photocatalytic degradation mechanism of zinc oxide is similar to titanium dioxide [18,19], so zinc oxide is regarded as a possible alternative to titanium dioxide. Some studies have shown that zinc oxide has a higher photocatalytic efficiency than titanium dioxide in degrading some hard-to-degrade organic compounds [20,21,22,23]. Zinc oxide is one of the important semiconductor materials of the II–VI family, which belongs to n-type semiconductor. Due to its excellent thermal stability and non-toxic properties, zinc oxide has been widely used in coatings, electronics, photocatalysis and sensor fields. In the field of photocatalysis [24,25], the band-gap energy of zinc oxide is 3.37 ev, which limits the most efficient catalytic degradation of zinc oxide under UV light and is less efficient under natural visible light. However, in the actual working environment, the continuous use of UV lamps is not as good as the use of natural visible light from the perspective of cost and environmental protection. Because the band gap energy of zinc oxide determines that it can only use the ultraviolet part of sunlight and the pimping proportion of ultraviolet light in natural light which is only 4%, the catalytic degradation efficiency of zinc oxide under visible light is not high. In order to improve the photocatalytic performance of zinc oxide, many attempts have been made, such as refining the grain size of zinc oxide [26], increasing the specific surface area [27,28], controlled microwave-assisted and pH-affected growth of zinc oxide [29] etc. At present, the most efficient method is element doping and recombination with precious metals or other semiconductors to improve the light response range of zinc oxide.
The specific degradation mechanism of zinc oxide is shown in the Figure 1. When zinc oxide absorbs light energy, the electrons transition from the valence band to the conduction band, because creating a photogenerated electron–hole pair in the zinc oxide crystal, electrons combine with adsorbed oxygen to form superoxide radicals, and holes combine with water molecules to form hydroxyl radicals, finally, highly oxidizing hydroxyl and superoxide radicals react with substances that need to be degraded, such as methyl blue and tetracycline, to turn them into harmless water and carbon dioxide.
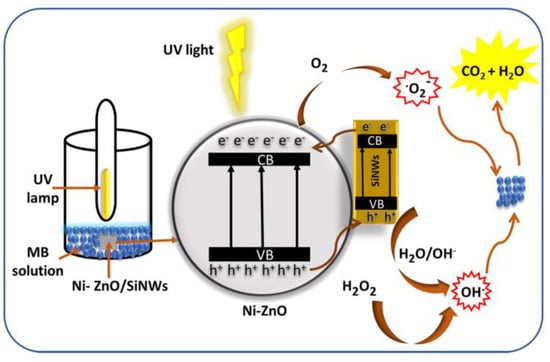
Figure 1. Photocatalysis of zinc oxide.
2. Zinc Oxide Doped with Transition Metal Ions
In recent years, scholars have mainly studied the doping of aluminum, nickel, iron, manganese, copper and other substances [35,36] on zinc oxide.
2.1. Doped with Divalent Metal Ions
Jannat Hammouche et al. [37] prepared nickel-doped zinc oxide by the sol-gel method, which controlled the content of nickel at 0~5%, then annealed it and coated the prepared nickel-zinc oxide spin onto the silicon nanowires to obtain zinc oxide-nickel/silicon nanowires. The effects of nickel doping on the structure, morphology and photocatalytic properties of ZnO-Ni/Si nanowires were studied. Photocatalytic studies showed that nickel doping slightly promoted the photocatalytic degradation of methylene blue, and the effect was the best when the amount of nickel doping was 5%, as Figure 2 shows.
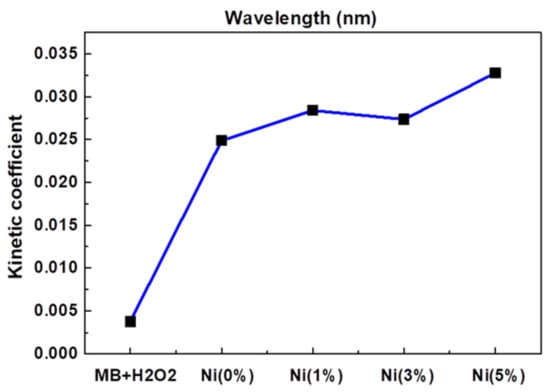
Figure 2. Kinetic constants of reaction between MB and ZnO doped with different Ni concentrations.
Lanqin Tang et al. [38] prepared zinc oxide photocatalysts with different co-doping degrees by the one-step dissolution method. It was found that the morphology of co-doped zinc oxide depended on the reaction temperature and the content of cobalt in solution. Additionally, it was found that the photocatalytic activity of cobalt-doped zinc oxide decreased, which might be due to the fact that the radius of cobalt and zinc ions are close to each other, thus forming a deep energy band gap level and reducing the photocatalytic activity. This indicates that doped transition metal ions are a double-edged sword, and further work can focus on the effects of continuous doping of different metal ions and their structures on the photocatalytic activity of zinc oxide [39], as Figure 2 shows.
2.2. Doped with Trivalent Metal Ions
Piangjai Peerakiatkhajohn et al. [40] used the sol-gel method to prepare low calcination temperature zinc oxide nanosheets and visible light-responsive Al-doped zinc oxide nanosheets. The results showed that calcination temperature and al doping had significant effects on the morphology, absorption properties and photocatalytic activity of zinc oxide nanoparticles. FE-SEM analysis showed that the spherical zinc oxide particles were transformed into nanorods and nanosheets at different calcination temperatures (200, 300 and 400 °C). At the same time, XRD analysis confirmed that the calcined zinc oxide nanosheets presented a hexagonal wurtzite structure, and with the increase of al doping amount, the absorption spectrum of al/zinc oxide nanosheets showed a red shift and the band gap became narrow, compared with the undoped zinc oxide nanosheets; it had excellent photocatalytic activity. The result showed that al doping can improve the photocatalytic activity of zinc oxide, as Figure 3 shows.
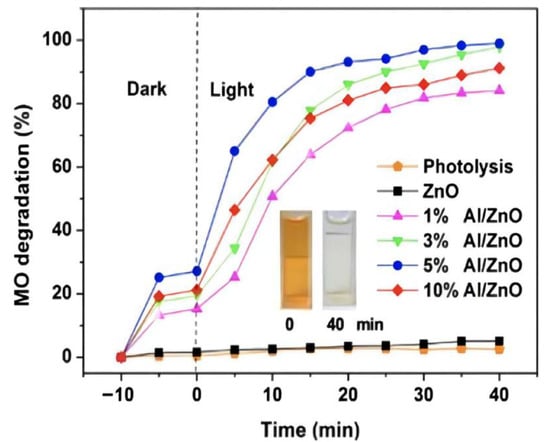
Figure 3. Photocatalytic efficiency of aluminum doping with different content (inset shows the color change in MO at time intervals 0 and 40 min).
Sabrina Roguai et al. [41] successfully prepared fe-containing zinc oxide nanoparticles by the chemical coprecipitation method. The method was low-cost and easy to realize. The structure of the synthesized zinc oxide powders was characterized by XRD, the results showed that the synthesized zinc oxide powders are of hexagonal lead-zinc ore structure. The SEM images showed that the size of nanoparticles was not uniform and agglomerate, and the grain size of doped sampled decreased with the increase of doping concentration. Pure zinc oxide nanopowders exhibit higher photocatalytic activity for the degradation of methylene blue dyes than iron-doped zinc oxide, as measured by photochemical properties [42]. Whether it is divalent metal ions or trivalent metal ions, the main thing to pay attention to being the concentration. Too low doping concentration has a poor effect and cannot improve the photocatalytic efficiency of zinc oxide to the maximum extent. If the concentration is too high, the second phase will appear in XRD detection and the internal structure of zinc oxide will be destroyed. Therefore, the concentration of doped metal ions is the part that needs to be measured many times to get the best ratio concentration [43,44,45].
2.3. Co-Doping of Metal Ions
In addition to single-doped metal ions, co-doped that more than two kinds of metal ions has become a popular research direction to improve the photocatalytic performance of zinc oxide.
Fatemeh Dabir et al. [46] prepared pure zinc oxide films and zinc oxide films doped with aluminum and copper by the sol-gel method, and studied their electro-optical properties. The experimental results showed that the size of Al and Cu-doped zinc oxide nanosheets had increased, and formed the cross-linked nanostructured film. At the same time, it was found that the substitution and gap filling of Al and Cu dopants led to instability of the zinc oxide crystal structure and to additional point defects, which have a significant effect on the photocatalytic properties and electrical conductivity of zinc oxide.
In addition, zinc oxide doped transition metal ions can also lead to the morphology of ZnO changed, such as doping aluminum can affect the internal stress of zinc oxide, cause the grain size of zinc oxide to be reduced and the specific surface area to be increased; it can also expose the more active site. As shown in Figure 4, abcd is, respectively, 1%, 3%, 5%, and 10% of Al doped ZnO in which it can be seen that the increase of Al content has a significant effect on the morphology of zinc oxide; nickel-doped zinc oxide particles were agglomerated into spheres, and copper and nickel-doped zinc oxide powders were reduced in size, agglomerated less, and dispersed better. These changes also affect the photocatalytic efficiency of zinc oxide. It is one of the effective ways to improve the photocatalytic efficiency of zinc oxide by doping metal ions.
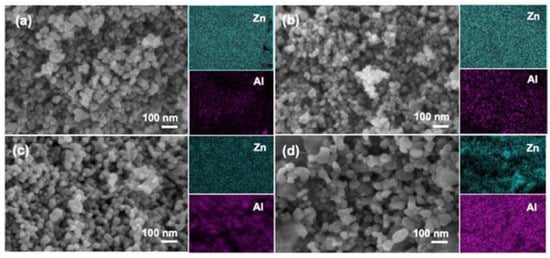
Figure 4. FE-SEM and elemental mapping images of: (a) 1% Al/ZnO; (b) 3% Al/ZnO; (c) 5% Al/ZnO; and (d) 10% Al/ZnO photocatalysts.
This entry is adapted from the peer-reviewed paper 10.3390/polym14214484
This entry is offline, you can click here to edit this entry!
