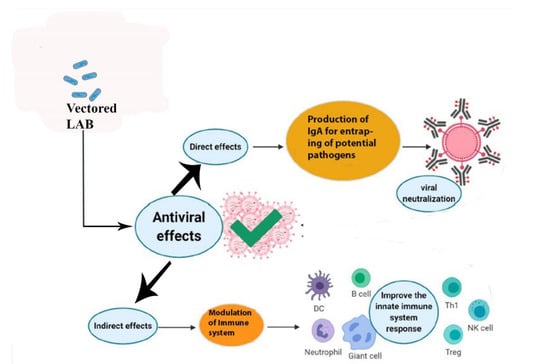
The immune system, which consists of the acquired and innate immune systems, works to neutralize invading viruses and other pathogens. Researchers reported that DCs play an important role in bridging innate and adaptive antiviral immunity. Numerous viruses are continually attacking the body. Epithelial surfaces, such as the skin and the mucosal linings of the digestive, respiratory, and urogenital tracts, which are home to DCs, are the first line of defense against pathogens, especially viruses [
15]. When these barriers are breached, pathogens are captured by DCs, which are activated and attach to lymphoid organs where the proper specialized immune responses are initiated [
15]. Mucosal immunity is the capacity to induce the protective immune response within mucosae where pathogens enter and initiate infections [
16,
17]. Animals and humans could initiate both systemic and mucosal immunity by recognizing pathogens as foreign objects for their neutralization. The difference between mucosal immunity and systemic immunity is the production of secretory immunoglobulin IgA (sIgA) which is more resistant to protease enzymes [
18,
19]. For protective mucosal immunity, participation of all kinds of mucosal immune cells are necessary for producing protective IgA antibodies. This process can be divided into entrance sites, where the pathogens adhere to the mucosal surface, and effector sites, where the plasma cells make antibodies that trigger a local immune response, as shown in
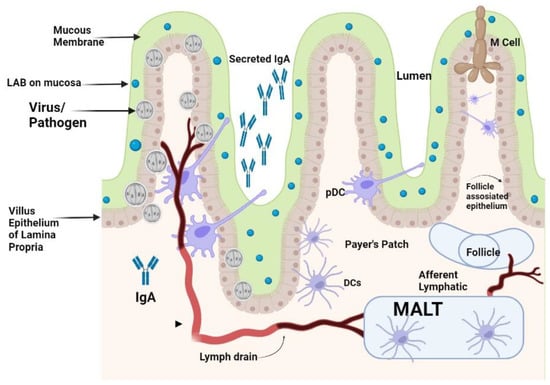
Figure 1 [
16,
20].
The LAB strains significantly impact on the process of DCs’ activation and the subsequent immunological responses. It has been demonstrated that murine DCs can respond differently depending on the strain of LAB, and this is exacerbated further by the fact that these responses can vary even amongst DC subtypes [
21,
22,
23]. It has been reported that
Lactobacillus modulates the maturation and function of DCs, macrophages, and CD
4+FoxP3
+ regulatory T cells (Tregs) as well as the differentiation of CD
4+CD
8+ and CD
4+FoxP3
+ T cells in Peyer’s patches (PPs) [
24,
25]. The counterattack of pathogens is carried out by specialized DCs of mucosa in the mesenteric lymph nodes also called membrane-associated lymphoid tissues (MALTs). These lymphoid tissues are located beneath the mucosal epithelium of the intestine. These MALTs are similar to peripheral lymph nodes with an abundant supply of B cells and M cells for capturing the invading pathogens [
26]. LAB could also initiate the cellular response by differentiation of DCs, and production of cytokines, that could favor the differentiation of näive T cells into Tregs, which are a specialized T cell subpopulation with specific regulatory mechanisms that inhibit the core components of adaptive and innate immune responses [
27]. Tregs can drive the depression of an excessive response of effector T cells either by Th1, Th2, or Th17 and maintain mucosal immune homeostasis [
10]. Differentiated DCs perform a significant role in the triggering of the immune system against challenging viruses by attaching to them. These DCs are mainly located in the MALTs of the mucosal membrane of the intestine along with some draining lymphoid nodes in the mucosal membrane of the gastrointestinal tract. Plasmacytoid DCs (pDCs) and conventional DCs (cDCs) are the types of DCs presenting at the mucosal membrane. The pDCs are less commonly found in the blood circulation, the mucosal membrane of GIT, and the lymphoid tissue that produces IFN-
α [
28]. The DCs in the mucosal membrane are classified into CX3CR1
+CD103
+ DCs with fractalkine (FKN) receptors and CX3CR1
+ DCs. Among these DCs, CX3CR1
+ DCs have long stellate extensions which elongate from epithelial cells to the antigen found in the lumen of the gut and they usually do not migrate to another place. The mucosal immunity is thought to be organized within the MALTs, thus the antigen must be transported from the lumen to the MALTs by DCs for Tregs to initiate the immune response [
29]. As a result of priming, a cascade of cytokines such as TGF-
β, IL-6, IL-10, IL-12, IL-23, and other molecules are produced. These cytokines started a cascade of other interleukins’ production and priming of T-helper cells to produce Th1, Th2, Th17, and other T regulatory cells for the neutralization of invading pathogens [
30]. Gut microbiota dysbiosis increases the susceptibility of an individual to various diseases. Emerging evidence suggests that LAB are beneficial for the control of RV and SARS-CoV-2 infections. Probiotics are known for restoring stable gut microbiota through the interactions and coordination of the intestinal innate and adaptive immunity [
31]. The researchers have reported on the effective protection of LAB against gastrointestinal viruses that originated from clinical cases in humans [
32]. The activation of antiviral peptides and the production of mucin by intestinal epithelial cells, and the activation of the local innate immune system lead to an increase in sIgA antibodies for neutralization of the challenge [
32]. RV infection deteriorates the mucosal barrier of the GIT [
33]. In the clinical cases of RV infection, when
Lacticaseibacillus rhamnosus GG (LGG) is administered orally, it could prohibit diarrhea caused by RV infection by mucosal immunity enhancement [
33]. The treated cases of LGG reduced the adverse effects of RV on the barrier function in the GIT of piglets, improved relatively the intestinal microbiota, lowered autophagy, increased apoptosis of epithelial cells in the ileum, and retarded the viral multiplication in the intestinal epithelial cells (IECs) [
33,
34]. It has also been shown that combinations of probiotics and immunization work together to effectively change the gut microbiota. An oral RV vaccine’s immunogenicity is increased by
Lactobacillus acidophilus, which also improves the production of IgG and IgA antibodies. Probiotics such as
Lactobacillus rhamnosus GG and
Bifidobacterium lactis Bb12 also modulate dendritic cell responses via distinct Toll-like receptor (TLR) signaling, and function as immunostimulants for the RV vaccine [
31].
LAB given orally travel down into the intestinal tract surviving through the stomach and are entrapped in the mucus layer secreted in the villi of the small intestine. Lactobacilli from enteric cells could come into contact with the mucosal epithelium. IgA that is secreted by sensed plasma cells in the epithelial membrane is secreted via the IgA receptor into the gut lumen and could be a superintended factor in bacterial presence. Pathogens that come in contact with the apical surface of the mucosal membrane might be sensed by DCs that can capture the viruses by entailment through their protrusions between enterocytes without breaching the integrity of the epithelial layer. The PPs found in the enteric wall are major contact sites where pathogens and antigens are prone to attach to enteric cells. M-cells in the epithelium transport pathogens present inside the lumen to the membrane-associated lymphoid tissue (MALTs) where pathogens are neutralized. DCs that are present in the area of the PPs can uptake and phagocytose viruses and transport them to the MALTs, where they can directly modulate immune responses that are activated by the potential pathogens.