Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Prostate cancer is the most common cancer and the second leading cause of cancer death in men. The imaging assessment and treatment of prostate cancer has vastly improved over the past decade. The introduction of PSMA PET-CT has improved the detection of loco-regional and metastatic disease. PSMA PET-CT also has a role in the primary diagnosis and staging, in detecting biochemical recurrence after curative treatment and in metastasis-directed therapy.
- prostate cancer
- imaging
- PSMA PET
1. Introduction
Since the advent of PSA testing, prostate cancer management has been fast evolving and heavily debated. This is in part due to the high prevalence but protracted course of the disease, coupled with the understanding of tumour biology—an enigma that is inherently limited by technology used to assess it. PSMA PET-CT is an example of technology that has influenced researcher's practice. It has resulted in a divergence from traditional algorithms, opened the realm of theranostics and highlighted as many uncertainties as it has improvements.
By all measures, PCa is the most common cancer in men and one of the most common cause of cancer deaths amongst men.[1][2] Routine PSA testing was met with resistance by opponents touting PCa is an indolent disease that men usually die with, rather than from.[3][4]This is not a surprising sentiment considering conflicting mortality benefits from screening and early treatment published in international literature[5][6][7] . Unfortunately, the resultant drops in PSA testing also correlated with increased prostate cancer mortality[8]. Furthermore, societal impact of men living with metastatic disease is unequivocal. Approximately, two million men each year are diagnosed with prostate cancer with 10 million men living with the disease and 700,000 of these living with metastatic disease[9]. Analysis of conflicting organisational policies and management algorithms requires historical examination of clinical practices.
Traditionally, prostate cancer risk stratification relied predominantly on PSA level & kinetics, digital rectal examination (DRE), and Gleason score obtained from non-targeted ‘sextant’ template biopsy[10]. PSA screening led to early treatment of prostate cancer, leading to a decline in mortality, but the limited specificity of PSA and DRE likely resulted in overdiagnosis and overtreatment[10]. Furthermore, the classic TRUS sextant biopsy probably missed a proportion of clinically significant cancers and detected cancers that researchers now know are unlikely to warrant immediate treatment[11]. In addition to a high overall error rate in accurate diagnosis of prostate cancer, staging of PCa was also limited by the poor accuracy of CT and bone scan. Prior to development of MRI and PSMA PET-CT, a variety of nomograms were then developed to help risk stratify patients diagnosed with PCa. For example, the NCCN guidelines use PSA, Gleason score, and clinical stage to stratify PCa into ‘very low’, ‘low’, ‘intermediate’, ‘high’, and ‘very high’ risk categories, which guide clinical management before and after definitive therapy[12]. Within the limits of the understanding then, changing nomenclatures and evolving nomograms sought to better guide researcher's practice.
With the same momentum, the focus partly diverged from the traditional categories and subclassifications, which are founded on light-microscopy glandular architecture and serum PSA levels[13]. The international community embarked on a deeper molecular and genetic understanding of PCa, the results of which are difficult to transplant into current practice[14]. Notably, a variety of serum, urine, and biopsy biomarkers have also been approved to more accurately identify men with high-grade PCa[15]. Similarly, imaging technology evolved to investigate more than cross-sectional architecture alone. The advent of multi-parametric MRI enriched the risk stratification algorithm in this way. Improved anatomical detail coupled with sequences used to differentiate tissue characteristics of internal structures has allowed targeted sampling that has been shown to yield more clinically significant disease[16][17]. Some have even proposed the combination of Pi-RADS score and mRNA urine test to improve PCa detection further [18].
2. Positron Emission Tomography-Computed Tomography (PET-CT)
PET began in the 1980s and employed the tracer-kinetic assay method and tomographic image reconstruction to provide images of ‘function’. The first PET scanners were built ‘in-house’ in various research institutions in the northern hemisphere. PET-CT was introduced as a clinical tool in late 2000 and replaced PET-only scanners. Positrons are positive electrons, which are emitted from the nucleus of positron emitters (18F, 15O, 11C, 13N), have short half-lives and are usually produced by medical cyclotrons. The positron emitters are ‘tagged’ or attached to compounds using complex synthetic modules to produce a PET radiopharmaceutical. The PET radiopharmaceutical then, usually after intravenous injection in humans in trace amounts (hence, the term ‘PET tracer’), participates but does not perturb, a biochemical process of interest such as glucose metabolism or receptor uptake. Positron emitters decay to a stable state by the emission of a positron, which when in tissue collides with an innocent bystander electron; this collision results in the annihilation of the electrons and the generation of two 511 keV gamma rays (photons) that are then detected by crystal detectors in the PET tomograph[19]. In prostate cancer, the most commonly used PET radiopharmaceutical is 68Ga-PSMA; 68Ga has a half-life of 68 min. 68Ga is usually produced from a 68Ga-68Ga generator with a few exceptions—researchers institution and a few other sites in the world produce 68Ga in a cyclotron[20]. In clinical practice, PET-CT imaging is usually undertaken at a set time, after the intravenous injection of the PET radiopharmaceutical, referred to as the ‘uptake time’. The uptake time for 68Ga-PSMA in researchers institution is 50 min. The uptake of the PET radiopharmaceutical in the tissue can be quantified by kinetic analysis but this requires continues scanning after the injection of the PET radiopharmaceutical and this consumes valuable time on the scanner. So, for the clinic, a semi-quantitative measurement of the uptake, the standardized uptake value (SUV), that relates uptake to the injected activity of the PET radiopharmaceutical, the patient’s weight and to time, is used.
Since 2000 there have been progressive improvements in the PET and CT technologies—smaller more efficient crystals, digital photomultiplier tubes in PET; faster CT scanners with 64- and 128-images slices; better reconstruction techniques, improvements in ‘time-of-flight’ timing and continuous bed motion. The most recent advance has been the development of long field of view PET-CT scanners or ‘total body’ scanners. Siemens Healthineers have introduced the Biograph Vision Quadra (the ‘Quadra’) a scanner with a 106 cm z-axis field-of-view (FOV) and United Imaging, the Explorer with a 200 cm FOV. Both scanners have markedly improved sensitivity enabling faster scans, lower injected doses of PET radiopharmaceuticals and superior image quality. There are less than a dozen such scanners in use at the present time. The majority of the images used in this study are from researchers Biograph Vision Quadra which was the 2nd such device installed and it went ‘live’ in May 2021. The sensitivity of the Quadra is 16× that of conventional PET-CT scanners and it allows the simultaneous acquisition of data throughout the z-axis extent of the scanner.
3. PSMA PET-CT
PSMA is a surface receptor antigen expressed in prostate tissue and tumour-associated neovasculature[21]. It is a glutamate carboxypeptidase type II non-secreted transmembrane protein comprising 750 amino acids. Antibodies that bind to the extracellular domain of PSMA were developed. The first was humanised IgG monoclonal antibody ‘J591′, followed by the DKFZ- PSMA-617 ligand and the peptide-linker unit DOTAGA-(I-y)fk (Sub-KuE), termed PSMA-I & T[22] and these were then radio-labelled. Most published studies report 68-Ga labelled compound but recently 18F-labelled PSMA have been used[23]. Of relevance to the interpretation of PSMA PET-CT scans, PSMA expression is found in normal prostatic tissue albeit to a mild degree, in the salivary and lacrimal glands, nasal space and larynx, liver, spleen, bowel, the kidneys and the sympathetic ganglia (Figure 1) (20). PSMA uptake is also found in other tumors including glioblastoma, thyroid, breast, lung, colon and renal carcinomas. PSMA uptake is also seen in benign tumors—haemangiomas, thyroid and adrenal adenomas, schwannomas and desmoid tumors—and also in reactive/inflammatory conditions and Paget’s disease[21]. PSMA is overexpressed in almost all PCa by around 100–1000 times the normal level, however the exact reason for this remains unclear[24][25]. Current hypothesis suggests that functionally PSMA has a role in folate metabolism, with the extra-cellular unit hydrolyzing glutamated folates released by PCa cells, which are then utilised to enhance proliferation in PCa cells[26]. It’s utility in the diagnosis and staging of prostate cancer has since become an extremely advantageous imaging modality furthering international urological practice.
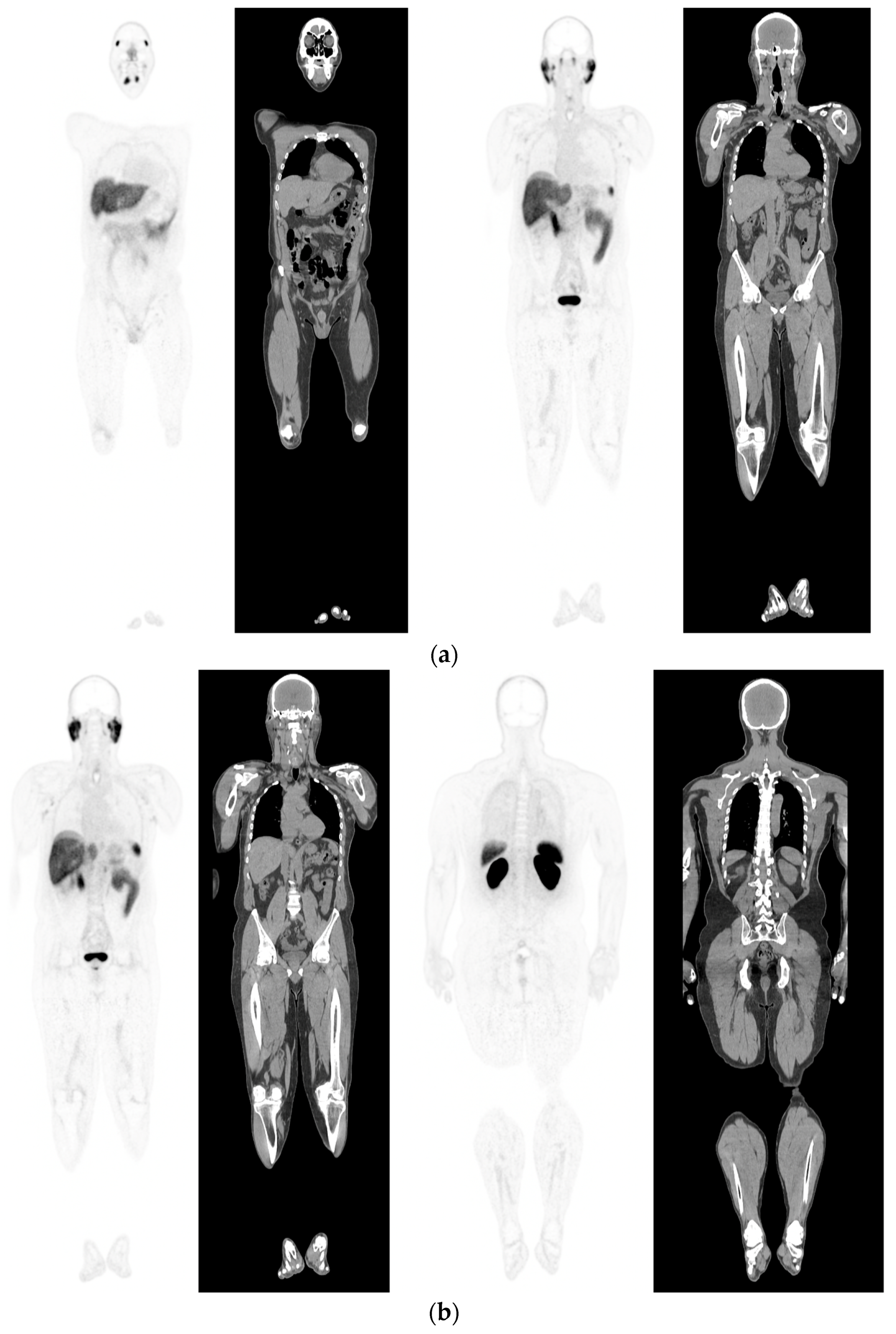
Figure 1. PSMA PET-CT scan showing normal distribution of PSMA-11. 60 yr old M—Gleason 6 prostate cancer on active surveillance for past 2 years; PSA 7.2; MR imaging—low grade changes PIRADS 2. PSMA PET-CT: 68Ga-PSMA - 204 MBq; uptake 52 min; BMI = 29.7; Wt 90 Kg; coronal PET and corresponding CT slices (soft tissue windows) from left to right. (a)—physiological uptake in lacrimal, submandibular salivary glands, parotid glands, retropharyngeal soft tissue, liver, bowel, part of spleen and pooling of tracer in bladder; mild reactive tracer uptake in groin and axillary nodes. (b)—physiological uptake in head and neck; reactive uptake axillary nodes; focal uptake in apex of prostate gland anterior below the bladder SUV = 9.1; marked uptake/excretion of tracer in both kidneys.
4. Metastatic PCa and PSMA PET-CT
European Association of Urology guidelines currently recommend the use of PSMA PET-CT for assessment of metastases[27]. In population-based studies PCa most commonly spreads to bone (84%), distant lymph nodes (10.6%), liver (10.2%), and thorax (9.1%)[28]. Approximately 18% of men have multiple metastatic sites involved (Figure 2). About 10% of patients with PCa have bone metastasis at presentation, and 33% of the remaining patients will develop metastases during follow-up[29]. Traditional imaging modalities may be helpful in evaluating distant metastases, with CT able to detect sclerotic bone lesions and visceral metastases, however CT has been reported to be positive in only 14% of cases[30]. BS has been up until recently the most widely used method to detect bone metastases in clinical practice due to its low cost. It can detect bone metastases with good sensitivity and can carry out whole body skeletal examination, however it is non-specific, with inflammation confounding metastatic deposits. Moreover, it reportedly only has a positive rate of 5% when PSA < 7 ng/mL[30], making it an imaging technique more suited to patients with very high PSA ranges and suspected late-stage disease.
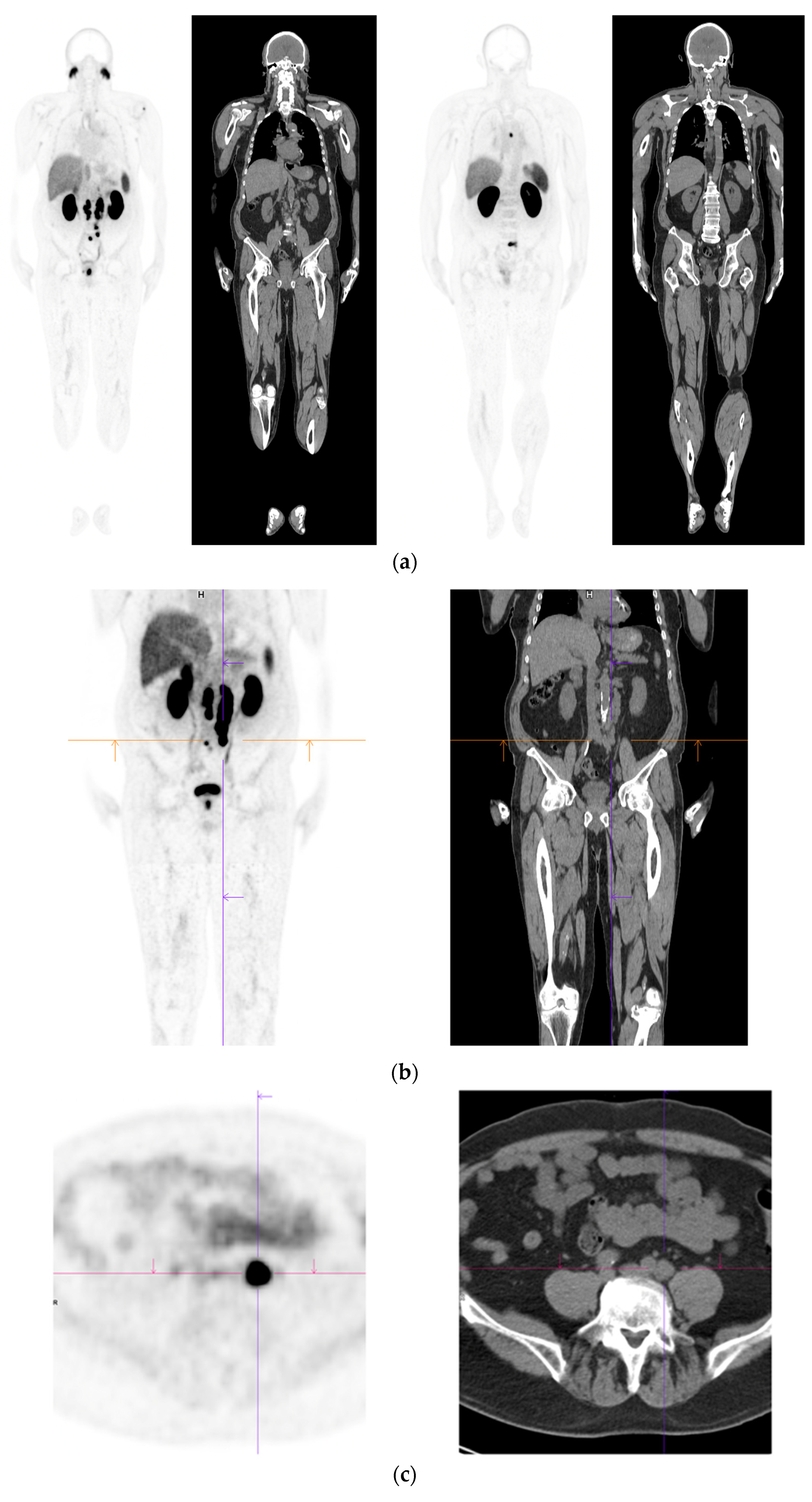
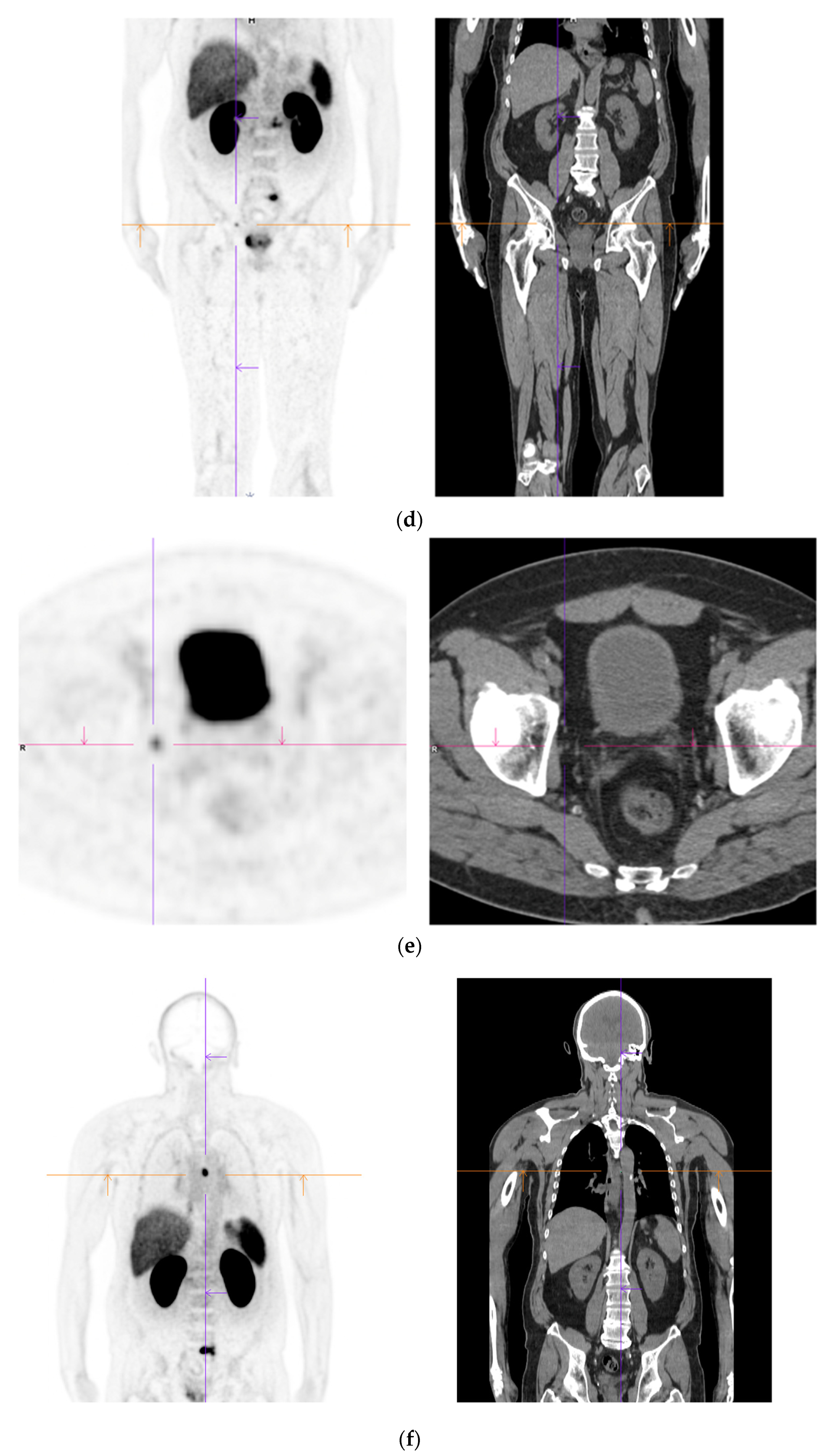
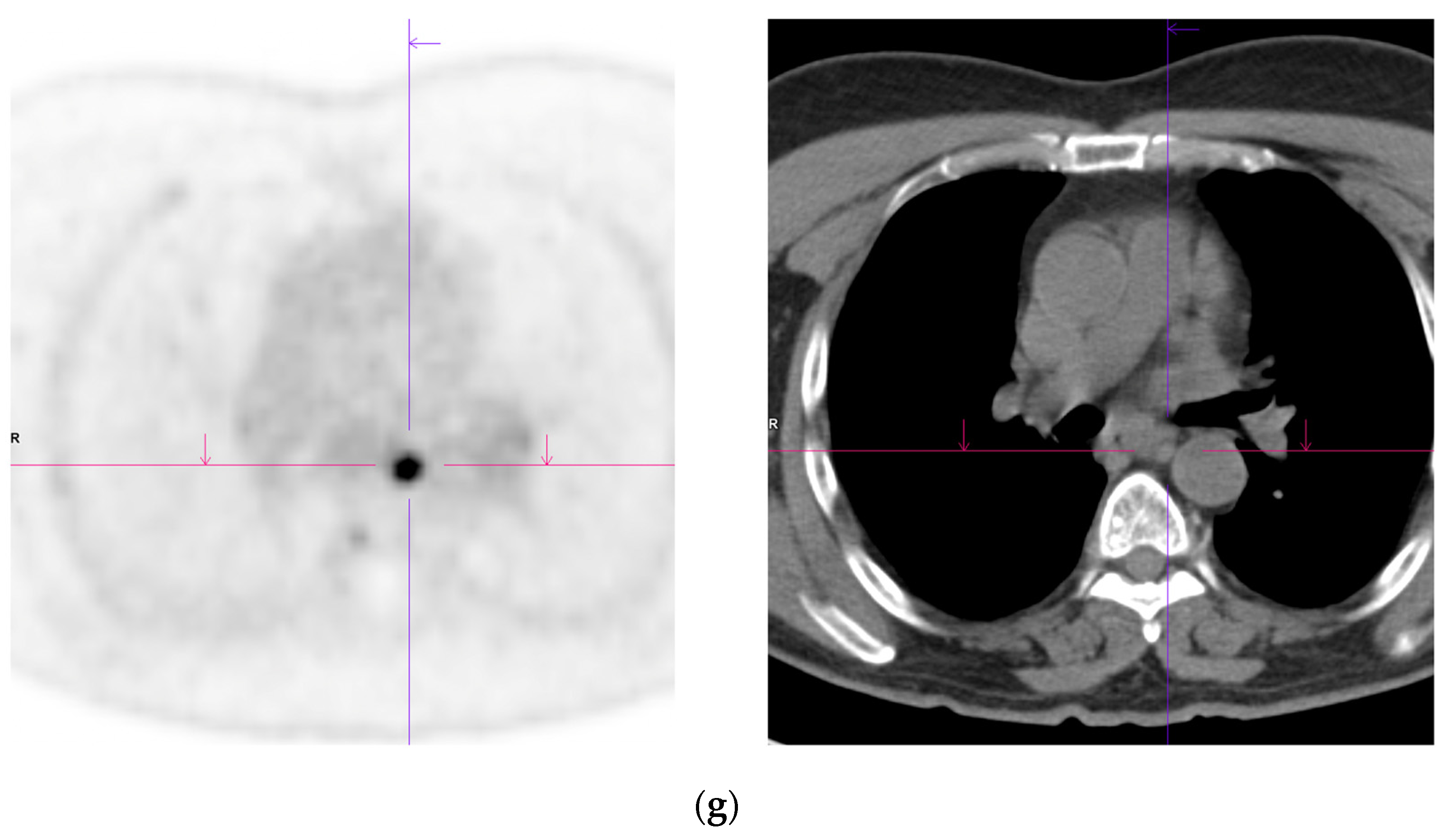
Figure 2. Recurrent disease. 74 yr old M; radical prostatectomy 8 yrs prior; extensive para-aortic and upper pelvic lymph node involvement on CT; severe back pain; PSA 60. PSMA PET-CT: 68Ga-PSMA-216 MBq; uptake 48 min; BMI = 31.9; Wt 89 kg. (a,b): Coronal and sagittal images show bulky nodal disease with markedly increased uptake (SUV = 68.2) in abdomen and pelvis, hepatic metastases, multiple bony metastases in pelvis, lower limbs, sternum, ribs and vertebral column. (c): enlarged transaxial images of lower thorax/upper abdomen show tracer avid foci in segments 7 (SUV = 8.8), 4 (SUV = 8.7) of liver; X-hairs on lesion in right 10th rib, SUV = 14.6; CT on bone windows shows small region of sclerosis vs. extensive uptake on PET.
A recent meta-analysis [31] aimed to compare the detection of bone metastases of PCa between PSMA PET-CT, NaF-PET-CT, choline-PET-CT, MRI and BS. They demonstrated PSMA and NaF-PET had higher pooled sensitivities on a per-patient basis (0.97 and 0.96 respectively) than choline-PET, MRI and BS (0.87, 0.91, 0.86). Further prospective trails have showed similar results with greater sensitivity (96% vs. 73%) and better specificity (99% vs. 84%) in the detection of skeletal metastases[32][33]. This improvement has profound implications in the management of small volume metastatic disease invisible on conventional staging.
Liver metastases typically occur in systemic, late stage disease, however there are cases of patients with liver metastases as the only metastatic site, thus early detection remains important for treatment decisions. There is evidence that PCa metastases to the liver are associated with neuroendocrine characteristic[34], and this malignant pattern might lead to the loss of PSMA-expression[35], which would hamper the visualisation of liver metastases by PSMA PET-CT. In a retrospective study, Damjanovic et al. (2019) reviewed 739 PCa patients for hepatic metastases using PSMA PET-CT together with CT or MRI. A total of 17 patients had hepatic metastases, with 15 patients (83.3%) demonstrating PSMA-positive metastases, two patients (11.1%) PSMA-negative metastases, and one patient (5.6%) had mixed metastases [36]. This study was limited by lack of histopathological confirmation of results as no liver biopsies were performed, however it shows that while PSMA PET-CT remains robust at 83.3% detection of PCa liver metastases, it’s limitation lies in the reliance of cellular expression of PSMA, which can be lost with disease progression and tumour dedifferentiation.
Pulmonary metastases are considered the second most common extra-nodal metastatic site for PCa in autopsy studies (lung 46% vs. bone 90%)[37], and its reliable detection as PCa, as opposed to a concurrent primary lung malignancy or benign process, is of high clinical importance for staging and management. Retrospective studies have found PSMA PET-CT to detect 72.5% of pulmonary metastases[38]. The PSMA PET-CT negative lesions (27.5%) were postulated to be secondary to loss of PSMA due to neuroendocrine transdifferentiation (confirmed histologically in a single case where the metastatic deposit was biopsied [38]), however Pryka et al., (2016) demonstrated that due to high PSMA uptake in lung cancer, PSMA PET-CT was unable to differentiate between a lung primary lesion and PCa metastasis[39]. The utility of PSMA PET-CT in assessment of lung lesions might be further restricted, as benign lesions such as areas of bronchiectasis[40], sarcoidosis[41] and tuberculosis[39].
Despite PSMA PET-CT improving the detection rate in early recurrence, there are clinical challenges to its use, primarily due to technical shortcomings including a short half-life (68Ga has a physical half-life of only 68 min [42]) and limited availability of 68Ga. It is known from PSMA PET-CT studies with different ligands that PCa lesions are shown with better contrast and higher tracer uptake after longer uptake times (eg. 3 h, rather than 1 h after injection which is the standard protocol)[43][44][45]. Thus, imaging with a ligand with longer half-life and higher activity (such as 18F(Fluorine)-PSMA-provides for higher lesion uptake and superior clearance of background activity. Interestingly, 18F-PSMA-1007 has also been found to have less urinary activity than PSMA PET-CT, which would improve its differentiation of local recurrence and regional lymph node metastases from ureter/bladder activity, and decrease rate of false positives[44][46].
Furthermore, as mentioned prior, PSMA is not exclusively expressed in PCa. This uptake in other parts of the body can potentially increase the difficulty of interpretation of PSMA PET-CT in suspected metastatic disease. A comprehensive prospective trial by Fendler et al., (2021) found that in patients post radiotherapy or RP who met criteria for BCR, (PSA > 0.2 mg/mL post RP or PSA > 2 ng/mL above nadir following radiation therapy) 17 of 217 patients (8%) had a false positive PSMA PET-CT Of these, almost two-thirds occurred in the context of suspected recurrence in the prostate post radiotherapy. Other causes for false positives included one case of primary lung cancer, one bronchogenic cyst, one prostatic abscess and two cases of fibrosis[47].
Another important consideration is the small proportion of reportedly negative PSMA PET-CT in the context of raised PSA[48] (e.g., PSA > 10 ng/mL, where negative PSMA PET-CT was 4%[49]) and metastatic hepatic and pulmonary lesions which are PSMA PET-CT negative. According to literature almost all prostatic adenocarcinomas will express PSMA[50], however there is a subpopulation that lacks strong PSMA tracer uptake, including men with neuroendocrine histology. Those men with advanced, castration resistant disease, may have areas of de-differentiation and loss of PSMA expression[25]. False negatives are also more common in patients with lower serum PSA values, or slower PSA kinetics. For purely intraductal carcinoma, which represents around 0.3% of all prostate cancers[51], the sensitivity of PSMA PET-CT has been questioned. Intraductal PCa has been shown to have a lower PSA expression by 30% and thus may make detecting intraductal PCa more difficult[52]. No specific studies have reviewed the efficacy of PSMA PET-CT in intraductal PCa, however several articles express concerns over their accuracy and suggest the addition of mpMRI or FDG PET to more accurately stage and monitor patients[53][54].
5. Stage Shift & Evolution of Oligometastatic Prostate Cancer
The current treatment paradigm for patients with rising PSA after maximal local therapies with negative conventional imaging is non-curative, consisting of systemic treatments. Amongst others, this algorithm is based on the results of the CHAARTED and LATITUDE trials, in which conventional imaging was used to detect metastatic disease[55][56][57]. In other words, patients with molecular PSMA-identified only oligo-recurrent or de novo synchronous oligometastatic disease were not included in these studies. Therefore, the recommendations of these seminal papers need to be interpreted carefully in patients with positive PSMA PET-CT but negative conventional imaging.
Similarly, many patients in previous literature considered to have high-risk localised disease were probably oligometastatic. While there is limited data in this space, researchers know that men with de novo oligometastatic disease in the H-arm of the STAMPEDE trial derived a 10% 3-year OS benefit from local radical radiotherapy in addition to systemic treatment, compared to those who received systemic treatment alone[58]. It is not unreasonable to extrapolate and expect similar outcomes for cytoreductive prostatectomy in this setting.
6. PSMA PET-CT and MDT (Metastasis Directed Therapy)
Metastatic PCa is becoming more accurately diagnosed and detected earlier through imaging such as PSMA PET-CT. Metastatic directed therapy is a newer concept aiming at improving outcomes for patients with oligometastases or metastatic disease. Historically, metastatic PCa was managed with chemotherapy, androgen biosynthesis inhibition, androgen receptor inhibition or radium 223[59]. However, several techniques have been established, specifically targeting metastases. Salvage ePLND has been shown to delay the development of a new clinical recurrence[60][61]. Yet, in studies with longer term follow >5 years, the efficacy and reduction in BCR is not as promising as once thought and therefore, salvage PLND should be perceived as a technique to delay BCR rather than a cure[62]. Essentially, salvage PLND is a form of metastectomy and cure for PCa is unlikely to be achieved with patients likely requiring salvage ADT and or chemotherapy and ultimately progressing towards CRPC.
Stereotactic Body Radiotherapy (SBRT) is another metastatic directed therapy which has been enhanced through the use of PSMA PET-CT. Several authors have shown higher disease free survival rates (64% vs. 34%), and lower long term requirement of ADT administration when using 68GA- PSMA PET vs. 18F-Choline for directed oligometastatic PCa treatment[63][64]. Similar benefits of using PSMA PET-CT to target skeletal oligometastatic disease have been demonstrated with over 40% of patients showing no evidence of disease progression[65]. Furthermore, elective nodal radiotherapy has also been shown to have a potential benefit for survival and decrease BCR in a recent systematic review. De Meerleer et al., (2021) found that patients with high risk PCa and evidence of pathologically positive pelvic lymph nodes predominately diagnosed through PSMA PET-CT had a substantial benefit with elective nodal radiotherapy, with minimal grade III or higher toxic effects[66].
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics12112594
References
- Siegel R.L, Miller K.D, Jemal A; Cancer Statistics. CA: a cancer journal for clinicans 2018, 68, 7-30, .
- Moyer V.A; Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement.. Ann Intern Med 2012, 157, 120-134, .
- T. R. A. C. o. G. Practitioners (2016) Guidelines for preventive activities in general practice. (RACGP).
- K. Pickles, S. M. Carter, L. Rychetnik, V. A. Entwistle, Doctors perspectives on PSA testing illuminate established differences in prostate cancer screening rates between Australia and the UK: a qualitative study. BMJ Open 6, e011932 (2016)
- G. L. Andriole et al., Mortality Results from a Randomized Prostate-Cancer Screening Trial. New England Journal of Medicine 360, 1310-1319 (2009).
- F. H. Schröder et al., Screening and Prostate-Cancer Mortality in a Randomized European Study. New England Journal of Medicine 360, 1320-1328 (2009).
- J. Hugosson et al., A 16-yr Follow-up of the European Randomized study of Screening for Prostate Cancer. Eur Urol 76, 43-51 (2019).
- S. L. James et al., Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet 392, 1789-1858 (2018).
- I. M. Thompson et al., Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst 98, 529-534 (2006).
- S. F. Shariat, C. G. Roehrborn, Using biopsy to detect prostate cancer. Rev Urol 10, 262-280 (2008).
- E. Schaeffer et al., NCCN Guidelines Insights: Prostate Cancer, Version 1.2021. J Natl Compr Canc Netw 19, 134-143 (2021).
- P. M. Pierorazio, P. C. Walsh, A. W. Partin, J. I. Epstein, Prognostic Gleason grade grouping: data based on the modified Gleason scoring system. BJU Int 111, 753-760 (2013).
- G. H. Leyten et al., Identification of a Candidate Gene Panel for the Early Diagnosis of Prostate Cancer. Clin Cancer Res 21, 3061-3070 (2015).
- L. Van Neste et al., Detection of High-grade Prostate Cancer Using a Urinary Molecular Biomarker-Based Risk Score. Eur Urol 70, 740-748 (2016).
- H. U. Ahmed et al., Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 389, 815-822 (2017).
- A. S. Purysko et al., PI-RADS Version 2.1: A Critical Review, From the AJR Special Series on Radiology Reporting and Data Systems. American Journal of Roentgenology 216, 20-32 (2020).
- O. Katzendorn et al., Combination of PI-RADS score and mRNA urine test—A novel scoring system for improved detection of prostate cancer. PLOS ONE 17, e0271981 (2022).
- A. Berger, How does it work? Positron emission tomography. Bmj 326, 1449 (2003).
- M. E. Rodnick et al., Cyclotron-based production of 68Ga, [68Ga]GaCl3, and [68Ga]Ga-PSMA-11 from a liquid target. EJNMMI Radiopharmacy and Chemistry 5, 25 (2020).
- D. A. Silver, I. Pellicer, W. R. Fair, W. D. Heston, C. Cordon-Cardo, Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res 3, 81-85 (1997).
- V. Nargund et al., Imaging with radiolabelled monoclonal antibody (MUJ591) to prostate‐specific membrane antigen in staging of clinically localized prostatic carcinoma: comparison with clinical, surgical and histological staging. BJU international 95, 1232-1236 (2005).
- R. A. Werner et al., (18)F-Labeled, PSMA-Targeted Radiotracers: Leveraging the Advantages of Radiofluorination for Prostate Cancer Molecular Imaging. Theranostics 10, 1-16 (2020).
- R. Zhao et al., The meta-analysis of the effect of 68Ga-PSMA-PET/CT diagnosis of prostatic cancer compared with bone scan. Medicine (Baltimore) 100, e25417 (2021).
- M. S. Hofman, R. J. Hicks, T. Maurer, M. Eiber, Prostate-specific Membrane Antigen PET: Clinical Utility in Prostate Cancer, Normal Patterns, Pearls, and Pitfalls. Radiographics 38, 200-217 (2018).
- D. S. OKeefe, D. J. Bacich, S. S. Huang, W. D. W. Heston, A Perspective on the Evolving Story of PSMA Biology, PSMA-Based Imaging, and Endoradiotherapeutic Strategies. J Nucl Med 59, 1007-1013 (2018).
- F. H. Drost et al., Prostate MRI, with or without MRI-targeted biopsy, and systematic biopsy for detecting prostate cancer. Cochrane Database Syst Rev 4, Cd012663 (2019).
- G. Gandaglia et al., Distribution of metastatic sites in patients with prostate cancer: A population-based analysis. Prostate 74, 210-216 (2014).
- V. Pasoglou et al., Pattern of metastatic deposit in recurrent prostate cancer: a whole-body MRI-based assessment of lesion distribution and effect of primary treatment. World J Urol 37, 2585-2595 (2019).
- C. J. Kane et al., Limited value of bone scintigraphy and computed tomography in assessing biochemical failure after radical prostatectomy. Urology 61, 607-611 (2003).
- X. Zhou et al., Evaluation of (18)F-PSMA-1007 PET/CT in prostate cancer patients with biochemical recurrence after radical prostatectomy. Transl Oncol 15, 101292 (2022).
- T. Lengana et al., (68)Ga-PSMA PET/CT Replacing Bone Scan in the Initial Staging of Skeletal Metastasis in Prostate Cancer: A Fait Accompli? Clin Genitourin Cancer 16, 392-401 (2018).
- N. Regula et al., Comparison of 68Ga-PSMA PET/CT with fluoride PET/CT for detection of bone metastatic disease in prostate cancer. European Journal of Hybrid Imaging 6, 5 (2022).
- D. Pouessel et al., Liver metastases in prostate carcinoma: clinical characteristics and outcome. BJU Int 99, 807-811 (2007).
- V. Parimi, R. Goyal, K. Poropatich, X. J. Yang, Neuroendocrine differentiation of prostate cancer: a review. Am J Clin Exp Urol 2, 273-285 (2014).
- J. Damjanovic et al., (68)Ga-PSMA-PET/CT for the evaluation of liver metastases in patients with prostate cancer. Cancer Imaging 19, 37 (2019).
- L. Bubendorf et al., Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol 31, 578-583 (2000).
- J. Damjanovic et al., (68) Ga-PSMA-PET/CT for the evaluation of pulmonary metastases and opacities in patients with prostate cancer. Cancer Imaging 18, 20 (2018).
- T. Pyka et al., 68Ga-PSMA-HBED-CC PET for Differential Diagnosis of Suggestive Lung Lesions in Patients with Prostate Cancer. J Nucl Med 57, 367-371 (2016).
- K. Bouchelouche, M. H. Vendelbo, Pulmonary Opacities and Bronchiectasis Avid on 68Ga-PSMA PET. Clin Nucl Med 42, e216-e217 (2017).
- P. J. Ardies et al., PSMA Uptake in Mediastinal Sarcoidosis. Clin Nucl Med 42, 303-305 (2017).
- C. Kesch, C. Kratochwil, W. Mier, K. Kopka, F. L. Giesel, (68)Ga or (18)F for Prostate Cancer Imaging? J Nucl Med 58, 687-688 (2017).
- A. Afshar-Oromieh et al., The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging 42, 197-209 (2015).
- K. Rahbar et al., (18)F-PSMA-1007 PET/CT at 60 and 120 minutes in patients with prostate cancer: biodistribution, tumour detection and activity kinetics. Eur J Nucl Med Mol Imaging 45, 1329-1334 (2018).
- A. Afshar-Oromieh et al., Radiation dosimetry of (68)Ga-PSMA-11 (HBED-CC) and preliminary evaluation of optimal imaging timing. Eur J Nucl Med Mol Imaging 43, 1611-1620 (2016).
- K. Rahbar et al., Advantage of (18)F-PSMA-1007 over (68)Ga-PSMA-11 PET imaging for differentiation of local recurrence vs. urinary tracer excretion. Eur J Nucl Med Mol Imaging 45, 1076-1077 (2018).
- W. P. Fendler et al., False positive PSMA PET for tumor remnants in the irradiated prostate and other interpretation pitfalls in a prospective multi-center trial. Eur J Nucl Med Mol Imaging 48, 501-508 (2021).
- B. Rosenzweig et al., Very Low Prostate PET/CT PSMA Uptake May Be Misleading in Staging Radical Prostatectomy Candidates. J Pers Med 12 (2022).
- A. Afshar-Oromieh et al., Diagnostic performance of (68)Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging 44, 1258-1268 (2017).
- J. S. Ross et al., Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin Cancer Res 9, 6357-6362 (2003).
- K. Watts, J. Li, C. Magi‐Galluzzi, M. Zhou, Incidence and clinicopathological characteristics of intraductal carcinoma detected in prostate biopsies: a prospective cohort study. Histopathology 63, 574-579 (2013).
- T. M. Morgan, C. J. Welty, F. Vakar-Lopez, D. W. Lin, J. L. Wright, Ductal adenocarcinoma of the prostate: increased mortality risk and decreased serum prostate specific antigen. J Urol 184, 2303-2307 (2010).
- N. Ranasinha et al., Ductal adenocarcinoma of the prostate: A systematic review and meta-analysis of incidence, presentation, prognosis, and management. BJUI Compass 2, 13-23 (2021).
- L. M. McEwan, D. Wong, J. Yaxley, Flourodeoxyglucose positron emission tomography scan may be helpful in the case of ductal variant prostate cancer when prostate specific membrane antigen ligand positron emission tomography scan is negative. J Med Imaging Radiat Oncol 61, 503-505 (2017).
- K. Fizazi et al., Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. The Lancet Oncology 20, 686-700 (2019).
- C. J. Sweeney et al., Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. New England Journal of Medicine 373, 737-746 (2015).
- C. E. Kyriakopoulos et al., Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J Clin Oncol 36, 1080-1087 (2018).
- C. C. Parker et al., Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet 392, 2353-2366 (2018).
- C. Henkenberens et al., Efficacy of PSMA PET-Guided Radiotherapy for Oligometastatic Castrate-Resistant Prostate Cancer. Frontiers in Oncology 11 (2021).
- D. K. Osmonov et al., Extended salvage pelvic lymph node dissection in patients with recurrent prostate cancer. Adv Urol 2014, 321619 (2014).
- K. Kolontarev et al., Extended robotic salvage lymphadenectomy in patients with node-only prostate cancer recurrence: initial experience. Cent European J Urol 71, 162-167 (2018).
- C. A. Bravi et al., Long-term Outcomes of Salvage Lymph Node Dissection for Nodal Recurrence of Prostate Cancer After Radical Prostatectomy: Not as Good as Previously Thought. Eur Urol 78, 661-669 (2020).
- R. Mazzola et al., Metastasis-directed Therapy (SBRT) Guided by PET-CT (18)F-CHOLINE Versus PET-CT (68)Ga-PSMA in Castration-sensitive Oligorecurrent Prostate Cancer: A Comparative Analysis of Effectiveness. Clin Genitourin Cancer 19, 230-236 (2021).
- X. Tu et al., The Role of 68Ga-PSMA Positron Emission Tomography/Computerized Tomography for Preoperative Lymph Node Staging in Intermediate/High Risk Patients With Prostate Cancer: A Diagnostic Meta-Analysis. Frontiers in Oncology 10 (2020).
- P. Rogowski et al., Outcomes of metastasis-directed therapy of bone oligometastatic prostate cancer. Radiation Oncology 16, 125 (2021).
- G. De Meerleer et al., Elective nodal radiotherapy in prostate cancer. The Lancet Oncology 22, e348-e357 (2021).
- W. Artibani, A. B. Porcaro, V. De Marco, M. A. Cerruto, S. Siracusano, Management of Biochemical Recurrence after Primary Curative Treatment for Prostate Cancer: A Review. Urologia Internationalis 100, 251-262 (2018).
This entry is offline, you can click here to edit this entry!
