Minimally invasive mitral valve surgery is evolving rapidly since the early 1990’s and is now increasingly adopted as the standard approach for mitral valve surgery. It has a long and challenging learning curve and there are many considerations regarding technique, planning and patient selection when starting a minimally invasive program.
- minimally invasive mitral valve surgery
- learning curve
- decision making
[1][2][3][4][5][6][7][8][9][10][11][12]
Abstract: Minimally invasive mitral valve surgery is evolving rapidly since the early 90's and is now increasingly adopted as the standard approach for mitral valve surgery. It has a long and challenging learning curve and there are many considerations regarding technique, planning and patient selection when starting a minimally invasive program. In the current review, we provide an overview of all considerations and the decision-making process during the learning curve.
1. Introduction
Minimally invasive mitral valve surgery (MIMVS) has evolved rapidly since the beginning in the early ’90 (1-3) and is now increasingly adopted as the standard approach for mitral valve surgery by many centers (4-10). The STS database showed an increase from 15,5% (2014) to 24,6% (2018) of all mitral valve procedures in the United States being approached by a less invasive access (11). In experienced centers, rates of minimally invasive access have been reported up to 74% (12) with good short- and long-term results in primary (4-6, 15) and in redo mitral valve surgery (7-10), equal repair rates as in sternotomy approach (13, 14).
2. Learning curve of minimally invasive mitral valve surgery
MIMVS has a long learning curve due to multiple factors complicating the learning process. The decrease of the surgical working space and remotely working with long shafted instruments while the surgical perspective is changed to a videoscopic view, is technically more demanding than working in a wide surgical field with shorter instruments. For the extra-corporeal circulation, the procedure requires peripheral canulation (femoral, axillary or direct aortic) with specific aortic clamp techniques (e.g. Chitwood clamp or endo-aortic balloon). There must be insight into the pitfalls for peripheral cannulation techniques. Adding more complex techniques, such as the endo-aortic balloon will require extra attention from the surgeon while there is already full focus needed for the surgical procedure.
The number of procedures required to complete the learning curve is reported at 75 – 125 cases and at least 2 surgeries per week to maintain optimal surgical outcome (12). To train thoracoscopic skills and surgical sequence of MIMVS, training modules and simulators are available to shorten the learning curve (16, 17).
3. Benefits and limitations of minimally invasive access
3.1. Benefits of minimally invasive access
In literature a range of advantages is described when mitral valve surgery is performed via minimally invasive access. A reduction of blood transfusions, post- operative atrial fibrillation, ventilation time, length of stay in intensive care unit or hospital and risk of deep sternal wound complications are reported for MIMVS (18, 19). In addition, minimally invasive access showed improved quality of life and faster return to daily activity compared to sternotomy in the early postoperative phase (first 3 months) (20, 21).
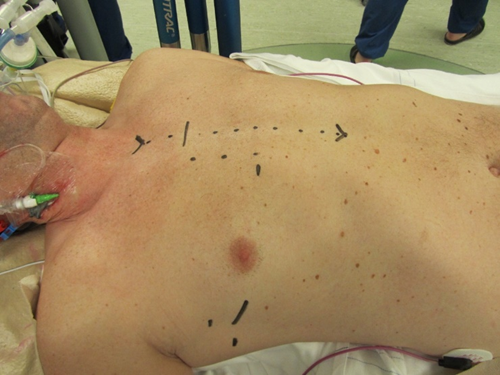
Figure 1. Marking of the incisions for MIMVS and the incision for conversion to sternotomy
3.2. Limitations of minimally invasive access
MIMVS is by no means a risk-free procedure, it has many advantages for the patients but it carries specific risks: a potentially higher stroke risk, groin complications, aortic dissection, longer cardiopulmonary bypass and aortic occlusion time are described. Concomitant procedures in MIMVS are usually limited to tricuspid valve surgery, closure of defects in the atrial septum and rhythm surgery. Chest deformations (e.g. pectus excavatum) or adhesions in the right thorax (e.g. prior pneumothorax or trauma) can limit the view in MIMVS.
4. Considerations in pre-operative planning and patient selection during the learning curve
4.1. Pre-operative planning and imaging
When starting a MIMVS program, careful patient selection and planning is necessary to establish a safe and successful program. A dedicated multidisciplinary mitral valve heart team can help select the best treatment (MIMVS vs. sternotomy vs. trans-catheter treatment vs. conservative) for each individual patient and is proven to improve long-term survival (22). In addition to the standard preoperative examinations (e.g. Chest X-ray, blood tests, transthoracic and/or trans-esophageal echocardiography and coronary angiogram), a thoracic CT scan and aortic CT from the femoral vessels to the aortic valve provide important information for surgical planning. Based on these images, calcifications in mitral annulus, thoracic deformations, the position of the mitral valve in the thoracic cavity and distance to the sternum is objectified and the optimal position of thoracic incision can be predicted.
4.2. Patient selection
Contraindications for MIMVS are safety related factors that expose unnecessary risks which are not in proportion with the benefits of less invasive access. Relative contraindications reported by the International Society for Minimally Invasive Cardiothoracic Surgery (ISMICS) are shown in table 1 (23).
Table 1. Relative contraindications and concerns for MIMVS reported by the International Society for Minimally Invasive Cardiothoracic Surgery (ISMICS).
|
Significant aortic, iliac, or femoral disease that prevents safe retrograde arterial perfusion |
|
Left ventricular ejection fraction < 25% |
|
Severe right ventricular dysfunction |
|
Pulmonary artery pressure > 70 mmHg |
|
Aorta > 4 cm if endo-aortic balloon being used |
|
Significant mitral annular calcification |
|
Patients with more than mild aortic regurgitation |
|
Kyphoscoliosis and pectus excavatum |
|
Morbidly obese and extremely muscular patients |
|
Previous right thoracotomy or expected adhesions in the right chest |
|
Advanced renal- or liver disease, significant pulmonary disease |
Table derived from Ailawadi et al. 2016 (33).
4.3. Considerations of mitral valve pathology during learning curve of MIMVS
Patients with complex repair, such as patients with anterior leaflet or bi-leaflet prolapse, should be avoided early in the learning curve as minimally invasive access should not compromise the outcome valve repair. Experienced centers have reported excellent long-term results of complex repairs (e.g. Barlow’s disease) through MIMVS with freedom from re-operation up to 93.8% in 10-years follow up and freedom from greater than 2+ grade mitral regurgitation up to 88.4% in 10- years follow up (24, 25).
5. Available techniques and safety considerations
5.1. Safety of the minimally invasive techniques
Multiple techniques are available at each step of surgery (e.g. chest access, cardiopulmonary bypass configuration, aortic occlusion). In the early days of MIMVS, there were concerns about the safety of this technique, especially with regard to aortic injury and stroke (19). Since the 1990s, results have improved significantly due to better pre-operative planning, adjustments to surgical technique and as more experienced centers have become familiar with the technique. A recent STS database study from 2021 and recent meta-analysis show similar results for MIMVS versus sternotomy (4-9, 11, 26, 27).

Figure 2. Setup of a MIMVS procedure by right anterolateral mini thoracotomy. Starting from the bottom of the soft tissue retractor in clockwise direction: Carbon dioxide insufflator, Transthoracic Chitwood clamp, video port and left atrial retractor.
The robotic assisted approach is the most complex to learn and has a learning curve beyond mini thoracotomy (28). In the robotic assisted technique, a 3D camera is used to visualize the chest and robotic arms are installed through ports and a variety of instruments can be inserted (e.g. scissors, diathermia, forceps).
5.3. Cardiopulmonary bypass: arterial cannulation
Cardiopulmonary bypass can be organized peripherally and centrally with a variety of configurations for many reasons. For peripheral cannulation, arterial access is most frequently obtained through the femoral artery, percutaneously (Seldinger technique) or via a groin incision, as it is a simple and reproducible technique that does not interfere with the surgical field. Whenever the femoral artery is inaccessible (e.g. in case of severe ilio-femoral calcifications, stenosis and tortuosity or thrombus in the thoraco-abdominal aorta), subclavian or central cannulation can be used. (29).
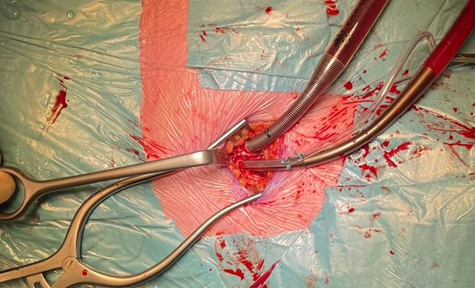
Figure 3. Peripheral arterial and venous cannulation in the right groin. Standard arterial cannula for direct aortic cross clamp technique. When using an endoaortic balloon, there is a sidearm in the arterial cannula to insert the balloon.
5.4. Cardiopulmonary bypass: Venous cannulation
As in arterial cannulation, peripheral venous cannulation is most frequently used in MIMVS. Access is obtained through the femoral vein, percutaneously (Seldinger technique) or through a groin incision, by inserting a two staged cannula positioned with the tip in the superior vena cava under TEE guidance, draining the superior and inferior caval vein. For tricuspid valve surgery or closure of an atrial septum defect, it is preferable to drain both caval veins separately, snaring both caval veins when opening the right atrium to prevent air lock in the extracorporeal circulation. When preferred an additional cannula can be inserted through the interna jugular or subclavian vein next to the femoral cannula (with the tip just below the diafragm) for separate caval venous drainage.
5.4. Cardiopulmonary bypass: Venous cannulation
As in arterial cannulation, peripheral venous cannulation is most frequently used in MIMVS. Access is obtained through the femoral vein, percutaneously (Seldinger technique) or through a groin incision, by inserting a two staged cannula positioned with the tip in the superior vena cava under TEE guidance, draining the superior and inferior caval vein. For tricuspid valve surgery or closure of an atrial septum defect, it is preferable to drain both caval veins separately, snaring both caval veins when opening the right atrium to prevent air lock in the extracorporeal circulation. When preferred an additional cannula can be inserted through the interna jugular or subclavian vein next to the femoral cannula (with the tip just below the diafragm) for separate caval venous drainage.
5.5. Techniques for aortic occlusion and de-airing
In literature, there are two techniques described for aortic cross clamping in MIMVS: the endo-aortic balloon occlusion and the direct transthoracic aortic clamp (e.g. Chitwood clamp).Compared to conventional heart surgery, the surgeon is most accustomed to the direct transthoracic aortic cross-clamp and is therefore most easy to handle (30). For the administration of cardioplegia in this technique, an aortic root vent must be placed. The use of the endo balloon was recently described in detail by van Praet et al (31, 32). In brief, the balloon occludes the aorta and cardioplegia is administered through the balloon. During the aortic occlusion, close monitoring of the pressures in the aortic root, the balloon itself and the radial artery pressure are important. Migration of the balloon is possible whenever there are changes in the pressures, such as during the start and stop of administration of cardioplegia, opening the left atrium and changes in cardiopulmonary bypass flow. In redo-surgery, the balloon can be very useful to occlude the aorta without necessity to perform adhesiolysis.
5.6. Hypothermic fibrillary arrest
In selected patients, such as in redo surgery (especially in patients with patent internal mammary artery grafts after CABG), a calcified ascending aorta without safe clamping site (hostile aorta) or whenever the endo-aortic balloon is contra indicated, hypothermic fibrillary arrest can provide safe myocardial preservation, however in patients with more than mild aortic valve regurgitation the vision can be hampered by retrograde blood.
5.7. Valve repair and replacement techniques
Repair rates of MIMVS have been reported to be comparable to sternotomy approach (13, 14). All techniques for mitral valve repair are feasible through minimally invasive access, although there is a trend towards ‘respect’ rather than ‘resect’ in MIMVS. Recent studies have shown that the neochord technique showed better long-term results than the resection technique in patients with degenerative disease who underwent mitral valve repair through minimally invasive access (33, 34).
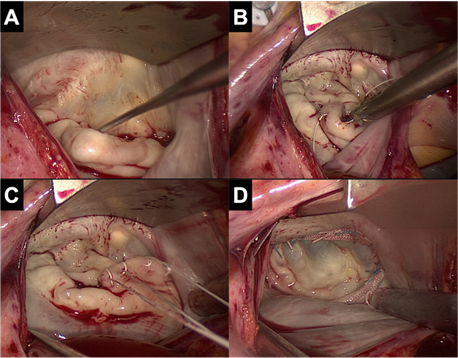
Figure 4. View on the mitral valve in MIMVS. A. Prolapse of P2 segment B. Placement of neochords on the prolapsing segment C. Adjusting of the height of the neochords D. Result of mitral valve repair
- Conversion to sternotomy
Conversion to sternotomy is reported at 1-2% in large series of experienced centers (4-6) and can be divided in an intended and unintended sternotomy. A perioperative unintended conversion is an emergency sternotomy due to a lesion of a major vascular structure or the heart (e.g. aortic dissection, atrioventricular dissociation). An intended conversion is a perioperative anticipated conversion to sternotomy due to inaccessible anatomy for MIMVS (e.g. unexpected peri-operative pulmonary adhesions).

Figure 5. Scar of minimally invasive access for mitral valve surgery
- Conclusion
MIMVS is increasingly adopted worldwide as the standard approach for mitral valve surgery due to less surgical trauma resulting in faster postoperative recovery and improved functional outcome. MIMVS is a complex technique with a long learning curve. The ideal case to start with is a mitral valve replacement or a straight forward mitral valve repair without pulmonary hypertension in a non-obese patient with good left ventricular function, no mitral calcification, no chest deformity, no previous cardiac surgery and no adhesions in the right chest. The most reproducible and simplest technique is access through right mini thoracotomy (totally thoracoscopic or with video assistance and direct view), cardiopulmonary bypass through peripheral femoral arterial and venous cannulation and aortic occlusion with a direct transthoracic clamp. The number required to complete the learning curve is currently reported at 75 – 125 cases and at least 2 cases per week to maintain optimal surgical outcome.
References
- Carpentier A, Loulmet D, Le Bret E, Haugades B, Dassier P, Guibourt P. [Open heart operation under videosurgery and minithoracotomy. First case (mitral valvuloplasty) operated with success]. C R Acad Sci III. 1996;319(3):219-23.
- Chitwood WR, Elbeery JR, Chapman WH, Moran JM, Lust RL, Wooden WA, et al. Video-assisted minimally invasive mitral valve surgery: the "micro-mitral" operation. J Thorac Cardiovasc Surg. 1997;113(2):413-4.
- Mohr FW, Falk V, Diegeler A, Walther T, van Son JA, Autschbach R. Minimally invasive port-access mitral valve surgery. J Thorac Cardiovasc Surg. 1998;115(3):567-74; discussion 74-6.
- Davierwala PM, Seeburger J, Pfannmueller B, Garbade J, Misfeld M, Borger MA, et al. Minimally invasive mitral valve surgery: "The Leipzig experience". Ann Cardiothorac Surg. 2013;2(6):744-50.
- Glauber M, Miceli A, Canarutto D, Lio A, Murzi M, Gilmanov D, et al. Early and long-term outcomes of minimally invasive mitral valve surgery through right minithoracotomy: a 10-year experience in 1604 patients. J Cardiothorac Surg. 2015;10:181.
- Ko K, de Kroon TL, Post MC, Kelder JC, Schut KF, Saouti N, et al. Minimally invasive mitral valve surgery: a systematic safety analysis. Open Heart. 2020;7(2).
- Ko K, de Kroon TL, Kelder JC, Saouti N, van Putte BP. Reoperative Mitral Valve Surgery Through Port Access. Semin Thorac Cardiovasc Surg. 2021.
- Seeburger J, Borger MA, Falk V, Passage J, Walther T, Doll N, et al. Minimally invasive mitral valve surgery after previous sternotomy: experience in 181 patients. Ann Thorac Surg. 2009;87(3):709-14.
- Murzi M, Miceli A, Di Stefano G, Cerillo AG, Farneti P, Solinas M, et al. Minimally invasive right thoracotomy approach for mitral valve surgery in patients with previous sternotomy: a single institution experience with 173 patients. J Thorac Cardiovasc Surg. 2014;148(6):2763-8.
- Romano MA, Haft JW, Pagani FD, Bolling SF. Beating heart surgery via right thoracotomy for reoperative mitral valve surgery: a safe and effective operative alternative. J Thorac Cardiovasc Surg. 2012;144(2):334-9.
- Nissen AP, Miller CC, 3rd, Thourani VH, Woo YJ, Gammie JS, Ailawadi G, et al. Less Invasive Mitral Surgery Versus Conventional Sternotomy Stratified by Mitral Pathology. Ann Thorac Surg. 2021;111(3):819-27.
- Holzhey DM, Seeburger J, Misfeld M, Borger MA, Mohr FW. Learning minimally invasive mitral valve surgery: a cumulative sum sequential probability analysis of 3895 operations from a single high-volume center. Circulation. 2013;128(5):483-91.
- Kastengren M, Svenarud P, Källner G, Franco-Cereceda A, Liska J, Gran I, et al. [13] initiating a minimally invasive programme. Eur J Cardiothorac Surg. 2020;58(6):1168-74.
- Gammie JS, Zhao Y, Peterson ED, O'Brien SM, Rankin JS, Griffith BP. J. Maxwell Chamberlain Memorial Paper for adult cardiac surgery. Less-invasive mitral valve operations: trends and outcomes from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg. 2010;90(5):1401-8, 10 e1; discussion 8-10.
- Luca F, van Garsse L, Rao CM, Parise O, La Meir M, Puntrello C, et al. Minimally invasive mitral valve surgery: a systematic review. Minim Invasive Surg. 2013;2013:179569.
- Jebran AF, Saha S, Waezi N, Al-Ahmad A, Niehaus H, Danner BC, et al. Design and training effects of a physical reality simulator for minimally invasive mitral valve surgery. Interact Cardiovasc Thorac Surg. 2019;29(3):409-15.
- Sardari Nia P, Daemen JHT, Maessen JG. Development of a high-fidelity minimally invasive mitral valve surgery simulator. J Thorac Cardiovasc Surg. 2019;157(4):1567-74.
- Cheng DC, Martin J, Lal A, Diegeler A, Folliguet TA, Nifong LW, et al. Minimally invasive versus conventional open mitral valve surgery: a meta-analysis and systematic review. Innovations (Phila). 2011;6(2):84-103.
- Falk V, Cheng DC, Martin J, Diegeler A, Folliguet TA, Nifong LW, et al. Minimally invasive versus open mitral valve surgery: a consensus statement of the international society of minimally invasive coronary surgery (ISMICS) 2010. Innovations (Phila). 2011;6(2):66-76.
- Moscarelli M, Lorusso R, Abdullahi Y, Varone E, Marotta M, Solinas M, et al. The Effect of Minimally Invasive Surgery and Sternotomy on Physical Activity and Quality of Life. Heart Lung Circ. 2021;30(6):882-7.
- Modi P, Hassan A, Chitwood WR, Jr. Minimally invasive mitral valve surgery: a systematic review and meta-analysis. Eur J Cardiothorac Surg. 2008;34(5):943-52.
- Sardari Nia P, Olsthoorn JR, Heuts S, van Kuijk SMJ, Vainer J, Streukens S, et al. Effect of a dedicated mitral heart team compared to a general heart team on survival: a retrospective, comparative, non-randomized interventional cohort study based on prospectively registered data. Eur J Cardiothorac Surg. 2021;60(2):263-73.
- Ailawadi G, Agnihotri AK, Mehall JR, Wolfe JA, Hummel BW, Fayers TM, et al. Minimally Invasive Mitral Valve Surgery I: Patient Selection, Evaluation, and Planning. Innovations (Phila). 2016;11(4):243-50.
- Borger MA, Kaeding AF, Seeburger J, Melnitchouk S, Hoebartner M, Winkfein M, et al. Minimally invasive mitral valve repair in Barlow's disease: early and long-term results. J Thorac Cardiovasc Surg. 2014;148(4):1379-85.
- Muneretto C, Bisleri G, Bagozzi L, Repossini A, Berlinghieri N, Chiari E. Results of minimally invasive, video-assisted mitral valve repair in advanced Barlow's disease with bileaflet prolapse. Eur J Cardiothorac Surg. 2015;47(1):46-50; discussion -1.
- Moscarelli M, Fattouch K, Gaudino M, Nasso G, Paparella D, Punjabi P, et al. Minimal Access Versus Sternotomy for Complex Mitral Valve Repair: A Meta-Analysis. Ann Thorac Surg. 2020;109(3):737-44.
- Barac YD, Glower DD. Port-Access Mitral Valve Surgery-An Evolution of Technique. Semin Thorac Cardiovasc Surg. 2020;32(4):829-37.
- Marin Cuartas M, Javadikasgari H, Pfannmueller B, Seeburger J, Gillinov AM, Suri RM, et al. Mitral valve repair: Robotic and other minimally invasive approaches. Prog Cardiovasc Dis. 2017;60(3):394-404.
- Lamelas J, Aberle C, Macias AE, Alnajar A. Cannulation Strategies for Minimally Invasive Cardiac Surgery. Innovations (Phila). 2020;15(3):261-9.
- Chitwood WR, Jr., Elbeery JR, Moran JF. Minimally invasive mitral valve repair using transthoracic aortic occlusion. Ann Thorac Surg. 1997;63(5):1477-9.
- Van Praet KM, Kofler M, Sundermann SH, Kempfert J. Endoaortic Balloon Occlusion During Minimally Invasive Mitral Valve Surgery. Innovations (Phila). 2022;17(2):83-7.
- Van Praet KM, Nersesian G, Montagner M, Akansel S, Eggert-Doktor D, Kofler M, et al. Endoaortic balloon occlusion in minimally invasive mitral valve surgery. Multimed Man Cardiothorac Surg. 2022;2022.
- Bonaros N, Hoefer D, Oezpeker C, Gollmann-Tepekoeylue C, Holfeld J, Dumfarth J, et al. Predictors of safety and success in minimally invasive surgery for degenerative mitral disease. Eur J Cardiothorac Surg. 2021.
- Pfannmueller B, Misfeld M, Verevkin A, Garbade J, Holzhey DM, Davierwala P, et al. Loop neochord versus leaflet resection techniques for minimally invasive mitral valve repair: long-term results. Eur J Cardiothorac Surg. 2021;59(1):180-6.
This entry is adapted from the peer-reviewed paper 10.3390/jcm11205993
References
- Carpentier, A; Loulmet, D; Le Bret, E e.a Open heart operation under videosurgery and minithoracotomie. First case (mitral valvuloplasty) operated with succes. C.R. Acad. Sci. 1996, 319, 219-223.
- W.Randolph Chitwood; Joseph R. Elbeery; William H.H. Chapman; Jon M. Moran; Robert L. Lust; William A. Wooden; David H. Deaton; Video-assisted minimally invasive mitral valve surgery: The “micro-mitral” operation. The Journal of Thoracic and Cardiovascular Surgery 1997, 113, 413-414, 10.1016/s0022-5223(97)70341-6.
- F.W. Mohr; V. Falk; A. Diegeler; T. Walther; J.A.M. van Son; R. Autschbach; Hans G. Borst; Minimally Invasive Port-Access Mitral Valve Surgery. The Journal of Thoracic and Cardiovascular Surgery 1998, 115, 567-576, 10.1016/s0022-5223(98)70320-4.
- Piroze M. Davierwala; Joerg Seeburger; Bettina Pfannmueller; Jens Garbade; Martin Misfeld; Michael A. Borger; Friedrich W. Mohr; Minimally invasive mitral valve surgery: “The Leipzig experience”. Annals of Cardiothoracic Surgery 2013, 2, 744-750, 10.3978/j.issn.2225-319X.2013.10.14.
- Mattia Glauber; Antonio Miceli; Daniele Canarutto; Antonio Lio; Michele Murzi; Daniyar Gilmanov; Matteo Ferrarini; Pier Andrea Farneti; Eugenio L. Quaini; Marco Solinas; et al. Early and long-term outcomes of minimally invasive mitral valve surgery through right minithoracotomy: a 10-year experience in 1604 patients. Journal of Cardiothoracic Surgery 2015, 10, 1-9, 10.1186/s13019-015-0390-y.
- Kinsing Ko; Thom L De Kroon; Marco C Post; Johannes C Kelder; Karen F Schut; Nabil Saouti; Bart P Van Putte; Minimally invasive mitral valve surgery: a systematic safety analysis. Open Heart 2020, 7, e001393, 10.1136/openhrt-2020-001393.
- Kinsing Ko; Thom L. de Kroon; Johannes C. Kelder; Nabil Saouti; Bart P. van Putte; Reoperative Mitral Valve Surgery Through Port Access. Seminars in Thoracic and Cardiovascular Surgery 2021, Online ahead of print., 0, 10.1053/j.semtcvs.2021.08.014.
- Joerg Seeburger; Michael A. Borger; Volkmar Falk; Jurgen Passage; Thomas Walther; Nicolas Doll; Friedrich W. Mohr; Minimally Invasive Mitral Valve Surgery After Previous Sternotomy: Experience in 181 Patients. The Annals of Thoracic Surgery 2009, 87, 709-714, 10.1016/j.athoracsur.2008.11.053.
- Michele Murzi; Antonio Miceli; Gioia Di Stefano; Alfredo G. Cerillo; Pierandrea Farneti; Marco Solinas; Mattia Glauber; Minimally invasive right thoracotomy approach for mitral valve surgery in patients with previous sternotomy: A single institution experience with 173 patients. The Journal of Thoracic and Cardiovascular Surgery 2014, 148, 2763-2768, 10.1016/j.jtcvs.2014.07.108.
- Matthew A. Romano; Jonathan W. Haft; Francis D. Pagani; Steven F. Bolling; Beating heart surgery via right thoracotomy for reoperative mitral valve surgery: A safe and effective operative alternative. The Journal of Thoracic and Cardiovascular Surgery 2012, 144, 334-339, 10.1016/j.jtcvs.2011.09.026.
- Alexander P. Nissen; Charles C. Miller; Vinod H. Thourani; Y. Joseph Woo; James S. Gammie; Gorav Ailawadi; Tom C. Nguyen; Less Invasive Mitral Surgery Versus Conventional Sternotomy Stratified by Mitral Pathology. The Annals of Thoracic Surgery 2020, 111, 819-827, 10.1016/j.athoracsur.2020.05.145.
- David M. Holzhey; Joerg Seeburger; Martin Misfeld; Michael A. Borger; Friedrich W. Mohr; Learning Minimally Invasive Mitral Valve Surgery. Circulation 2013, 128, 483-491, 10.1161/circulationaha.112.001402.
- Mikael Kastengren; Peter Svenarud; Göran Källner; Anders Franco-Cereceda; Jan Liska; Isak Gran; Magnus Dalén; Minimally invasive versus sternotomy mitral valve surgery when initiating a minimally invasive programme. European Journal of Cardio-Thoracic Surgery 2020, 58, 1168-1174, 10.1093/ejcts/ezaa232.
