The genus Euglena contains unicellular eukaryotic flagellates. In addition to light and chemicals, the cells perceive the gravitational field of the Earth and orient themselves paralell to the gravivector to optimize their position in the water column. The perception is based on transient receptor proteins in the membrane which are stimulated by the pressure of the cell content onto the lower membrane. Upon stimulation these proteins open and allow the influx of calcium from the outer medium which binds to a specific calmodulin. In turn this enzyme activates a adenylyl cyclase which produces cAMP believed to stimulate a phosphodiesterase A which finally modifies a flagellar protein which results in a course correction. Since Euglena is photosynthetic it absorbs carbon dioxide and produces oxygen and is thus an excellent candidate for a bioregenerative life support system during long-term space flights.
- flagellate
- gravitaxis
- graviperception
1. Characteristics of the Genus Euglena
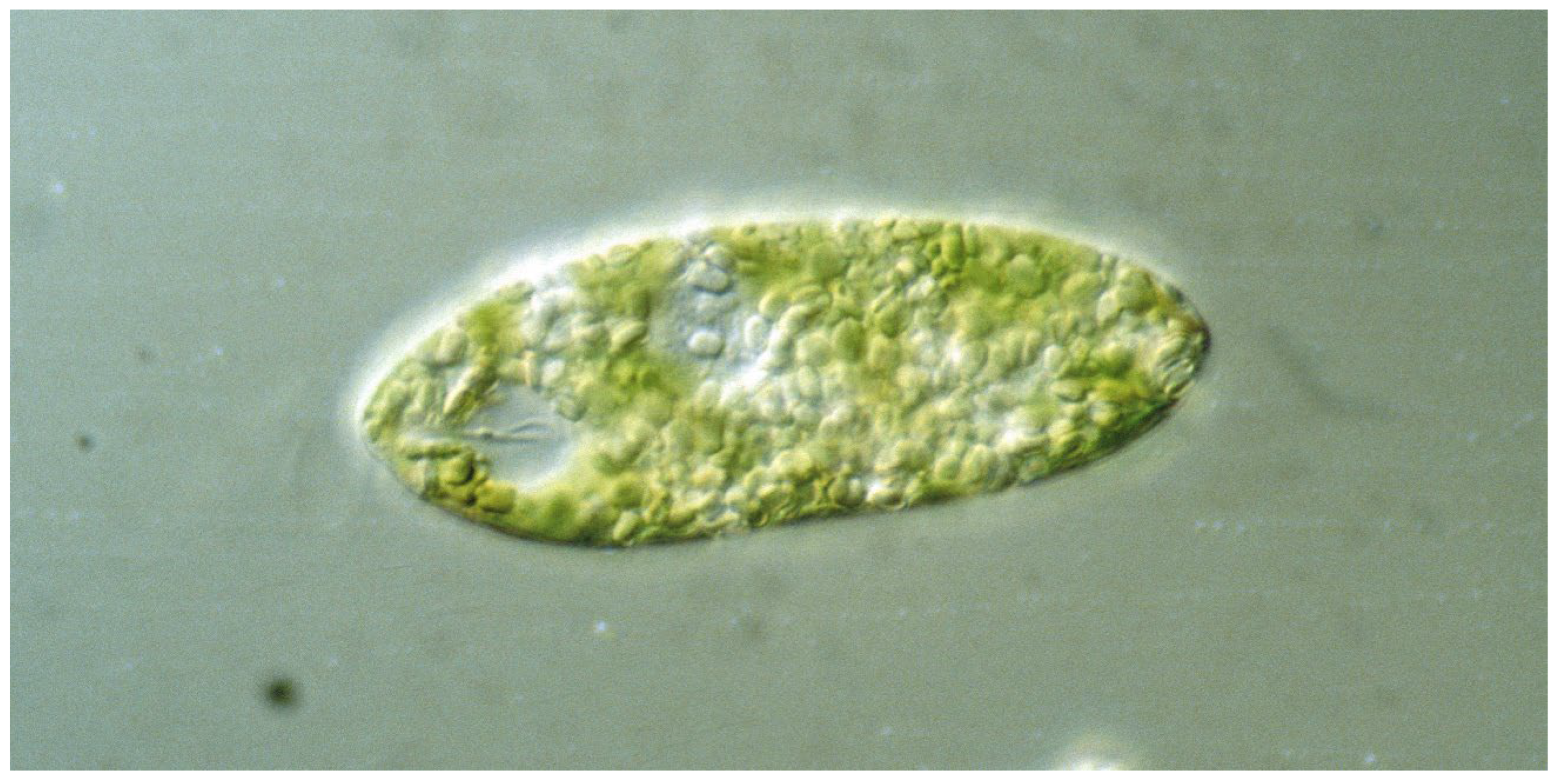
2. Graviperception and Graviresponses
Physiology of Gravitaxis
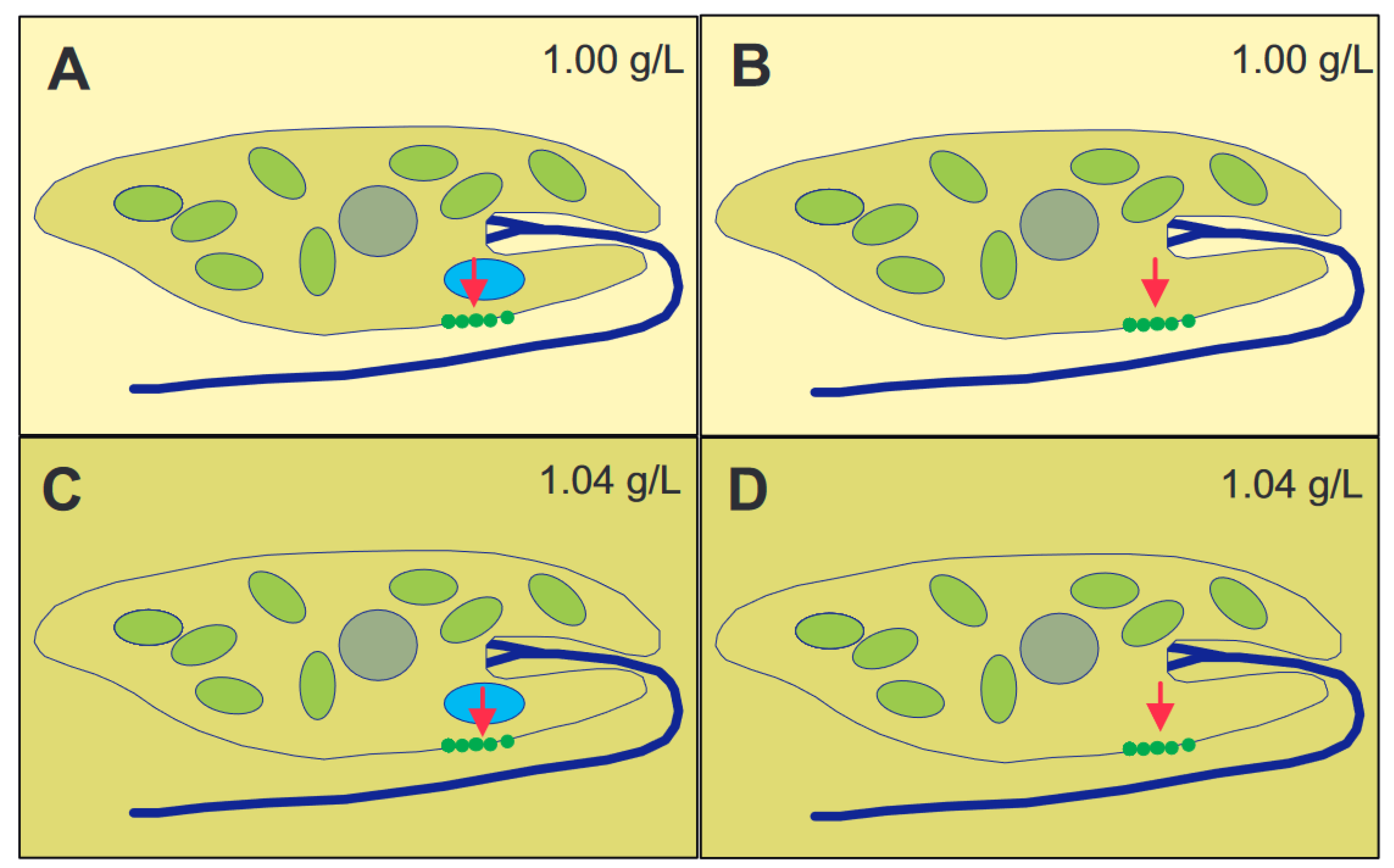
This entry is adapted from the peer-reviewed paper 10.3390/life12101522
References
- Oliva-Martínez, M.G.; Godínez-Ortega, J.L.; Zuñiga-Ramos, C.A. Biodiversidad del fitoplancton de aguas continentales en México. Rev. Mex. Biodivers. 2014, 85, 54–61.
- Häder, D.-P.; Hoiczyk, E. Gliding motility. In Algal Cell Motility; Melkonian, M., Ed.; Current Phycology; Chapman and Hall: New York, NY, USA, 1992; pp. 1–38.
- Häder, D.-P.; Melkonian, M. Phototaxis in the gliding flagellate, Euglena mutabilis. Arch. Microb. 1983, 135, 25–29.
- Wolken, J.J. Euglena: The photoreceptor system for phototaxis. J. Protozool. 1977, 24, 518–522.
- Ligęza, S.; Wilk-Woźniak, E. The occurrence of a Euglena pascheri and Lepocinclis ovum bloom in an oxbow lake in southern Poland under extreme environmental conditions. Ecol. Indic. 2011, 11, 925–929.
- Chaudhuri, D.; Ghate, N.B.; Deb, S.; Panja, S.; Sarkar, R.; Rout, J.; Mandal, N. Assessment of the phytochemical constituents and antioxidant activity of a bloom forming microalgae Euglena tuba. Biol. Res. 2014, 47, 1–11.
- Gerber, S.; Häder, D.-P. Effects of enhanced UV-B irradiation on the red coloured freshwater flagellate Euglena sanguinea. FEMS Microbiol. Ecol. 1994, 13, 177–184.
- Kings, A.J.; Raj, R.E.; Miriam, L.M.; Visvanathan, M.A. Cultivation, extraction and optimization of biodiesel production from potential microalgae Euglena sanguinea using eco-friendly natural catalyst. Energy Convers. Manag. 2017, 141, 224–235.
- Sato, N. Complex origins of chloroplast membranes with photosynthetic machineries: Multiple transfers of genes from divergent organisms at different times or a single endosymbiotic event? J. Plant Res. 2020, 133, 15–33.
- Zakryś, B.; Milanowski, R.; Karnkowska, A. Evolutionary origin of Euglena. In Euglena: Biochemistry, Cell and Molecular Biology; Schwartzbach, S., Shigeoka, S., Eds.; Springer: Cham, Switzerland, 2017; pp. 3–17.
- Lebert, M.; Häder, D.-P. Behavioral mutants of Euglena gracilis: Functional and spectroscopic characterization. J. Plant Physiol. 1997, 151, 188–195.
- Ozasa, K.; Kang, H.; Song, S.; Tamaki, S.; Shinomura, T.; Maeda, M. Regeneration of the eyespot and flagellum in Euglena gracilis during cell division. Plants 2021, 10, 2004.
- Basterretxea, G.; Font-Munoz, J.S.; Tuval, I. Phytoplankton orientation in a turbulent ocean: A microscale perspective. Front. Mar. Sci. 2020, 7, 185.
- Brodhun, B.; Häder, D.-P. Photoreceptor proteins and pigments in the paraflagellar body of the flagellate Euglena gracilis. Photochem. Photobiol. 1990, 52, 865–871.
- Häder, D.-P.; Iseki, M. Photomovement in Euglena. In Euglena: Biochemistry, Cell and Molecular Biology; Schwartzbach, S., Shigeoka, S., Eds.; Springer: Cham, Switzerland, 2017; pp. 207–235.
- Richter, P.R.; Streb, C.; Häder, D.-P. Sign change of phototaxis in Euglena gracilis. Trends Photochem. Photobiol. 2006, 11, 57–61.
- Iseki, M.; Matsunaga, S.; Murakami, A.; Ohno, K.; Shiga, K.; Yoshida, C.; Sugai, M.; Takahashi, T.; Hori, T.; Watanabe, M. A blue-light-activated adenylyl cyclase mediates photoavoidance in Euglena gracilis. Nature 2002, 415, 1047–1051.
- Ntefidou, M.; Iseki, M.; Watanabe, M.; Lebert, M.; Häder, D.-P. Photoactivated adenylyl cyclase controls phototaxis in the flagellate Euglena gracilis. Plant Physiol. 2003, 133, 1517–1521.
- Häder, D.-P.; Ntefidou, M.; Iseki, M.; Watanabe, M. Phototaxis photoreceptor in Euglena gracilis. In Light Sensing in Plants; Wada, M., Shimazaki, K., Iino, M., Eds.; Springer: Tokyo, Japan, 2005; pp. 223–229.
- Ozasa, K.; Won, J.; Song, S.; Maeda, M. Behavior of Euglena gracilis under simultaneous competing optical and chemical stimuli. Algal Res. 2018, 35, 98–105.
- Checcucci, A.; Colombetti, G.; del Carratore, G.; Ferrara, R.; Lenci, F. Red light induced accumulation of Euglena gracilis. Photochem. Photobiol. 1974, 19, 223–226.
- Tanimoto, Y.; Izumi, S.; Furuta, K.; Suzuki, T.; Fujiwara, Y.; Fujiwara, M.; Hirata, T.; Yamada, S. Effects of high magnetic field on Euglena gracilis. Int. J. Appl. Electromagn. Mech. 2002, 14, 311–316.
- Lebert, M.; Richter, P.; Porst, M.; Häder, D.-P. Mechanism of gravitaxis in the flagellate Euglena gracilis. In Proceedings of the 12th C.E.B.A.S.Workshops. Annual Issue 1996, Bochum, Germany; 1996; pp. 225–234.
- Häder, D.-P.; Lebert, M.; Richter, P. Gravitaxis and graviperception in Euglena gracilis. Adv. Space Res. 1998, 21, 1277–1284.
- Ullrich, O.; Häder, D.-P. Editorial. Signal transduction in gravity perception: From microorganisms to mammals. Signal Transduct. 2006, 6, 377–379.
- Hock, B.; Häder, D.-P. Graviresponses in fungi and slime molds. Signal Transduct. 2006, 6, 443–448.
- Hemmersbach, R.; Volkmann, D.; Häder, D.-P. Graviorientation in protists and plants. J. Plant Physiol. 1999, 154, 1–15.
- Ullrich, O.; Thiel, C.S. Gravitational Force: Triggered stress in cells of the immune system. In Stress Challenges and Immunity in Space; Springer: Berlin, Germany, 2012; pp. 187–202.
- Platt, J.B. On the specific gravity of Spirostomum, Paramecium and the tadpole in relation to the problem of geotaxis. Am. Nat. 1899, 33, 31.
- Köhler, O. Über die Geotaxis von Paramecium. Verh. Dtsch. Zool. Ges. 1921, 26, 69–71.
- Dryl, S. Behavior and motor response of Paramecium. In Paramecium: A Current Survey; van Wagtendonk, W.J., Ed.; Elsevier Scientific: Amsterdam, The Netherlands, 1974; pp. 165–218.
- Haupt, W. Geotaxis. In Handbuch der Pflanzenphysiologie; Ruhland, W., Ed.; Springer: Berlin/Heidelberg, Germany, 1962; Volume 17/2, pp. 390–395.
- Kuznicki, L. Behavior of Paramecium in gravity fields. I. Sinking of immobilized specimens. Acta Protozool. 1968, 6, 109–117.
- Hemmersbach, R.; Voormanns, R.; Häder, D.-P. Graviresponses in Paramecium biaurelia under different accelerations: Studies on the ground and in space. J. Exp. Biol. 1999, 390, 2199–2205.
- Machemer, H.; Bräucker, R. Gravireception and graviresponses in ciliates. Acta Protozool. 1992, 31, 185–214.
- Fukui, K.; Asai, H. Negative geotactic behavior of Paramecium caudatum is completely described by the mechanism of buoyancy-oriented upward swimming. Biophys. J. 1985, 47, 479–482.
- Grolig, F.; Herkenrath, H.; Pumm, T.; Gross, A.; Galland, P. Gravity susception by buoyancy: Floating lipid globules in sporangiophores of Phycomyces. Planta 2004, 218, 658–667.
- Grolig, F.; Döring, M.; Galland, P. Gravisusception by buoyancy: A mechanism ubiquitous among fungi? Protoplasma 2006, 229, 117–123.
- Häder, D.-P.; Lebert, M. Photoorientation in photosynthetic flagellates. In Methods in Molecular Biology; Jin, T., Hereld, D., Eds.; Humana Press: Clifton, NJ, USA, 2009; Volume 571, pp. 51–65.
- Stallwitz, E.; Häder, D.-P. Motility and phototactic orientation of the flagellate Euglena gracilis impaired by heavy metal ions. J. Photochem. Photobiol. B Biol. 1993, 18, 67–74.
- Stallwitz, E.; Häder, D.-P. Effects of heavy metals on motility and gravitactic orientation of the flagellate, Euglena gracilis. Eur. J. Protistol. 1994, 30, 18–24.
- Richter, P.R.; Ntefidou, M.; Streb, C.; Faddoul, J.; Lebert, M.; Häder, D.-P. High light exposure leads to a sign change of gravitaxis in the flagellate Euglena gracilis. Acta Protozool. 2002, 41, 343–351.
- Ntefidou, M.; Richter, P.; Streb, C.; Lebert, M.; Häder, D.-P. High light exposure leads to a sign change in gravitaxis of the flagellate Euglena gracilis. In Proceedings of the Life in Space for Life on Earth. 8th European Symposium on Life Sciences Research in Space. 23rd Annual International Gravitational Physiology Meeting, Karolinska Institutet, Stockholm, Sweden, 2–7 June 2002; pp. 301–302.
- Richter, P.R.; Streb, C.; Ntefidou, M.; Lebert, M.; Häder, D.-P. High light-induced sign change of gravitaxis in the flagellate Euglena gracilis is mediated by reactive oxygen species. Acta Protozool. 2003, 42, 197–204.
- Häder, D.-P.; Liu, S.M. Motility and gravitactic orientation of the flagellate, Euglena gracilis, impaired by artificial and solar UV-B radiation. Curr. Microbiol. 1990, 21, 161–168.
- Richter, P.; Börnig, A.; Streb, C.; Ntefidou, M.; Lebert, M.; Häder, D.-P. Effects of increased salinity on gravitaxis in Euglena gracilis. J. Plant Physiol. 2003, 160, 651–656.
- Lebert, M.; Häder, D.-P. Negative gravitactic behavior of Euglena gracilis can not be described by the mechanism of buoyancy-oriented upward swimming. Adv. Space Res. 1999, 24, 851–860.
- Staves, M.P. Cytoplasmic streaming and gravity sensing in Chara internodal cells. Planta 1997, 203, 79–84.
- Gadalla, D.; Braun, M.; Böhmer, M. Gravitropism in higher plants: Cellular aspects. In Gravitational Biology—Gravity Sensing and Graviorientation in Microorganisms and Plants; Braun, M., Häder, D.-P., Böhmer, M., Palme, K., Hemmersbach, R., Eds.; Springer: Cham, Switzerland, 2018.
- Sack, F.D. Plastids and gravitropic sensing. Planta 1997, 203, 63–68.
- Schnabl, H. Gravistimulated effects in plants. In Astrobiology; Horneck, G., Baumstark-Khan, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2002.
- Lebert, M.; Porst, M.; Richter, P.; Häder, D.-P. Physical characterization of gravitaxis in Euglena gracilis. J. Plant Physiol. 1999, 155, 338–343.
- Lebert, M.; Häder, D.-P. How Euglena tells up from down. Nature 1996, 379, 590.
- Lebert, M.; Richter, P.; Häder, D.-P. Signal perception and transduction of gravitaxis in the flagellate Euglena gracilis. J. Plant Physiol. 1997, 150, 685–690.
- Häder, D.-P.; Hemmersbach, R.; Lebert, M. Gravity and the Behavior of Unicellular Organisms; Cambridge University Press: Cambridge, UK, 2005; pp. 1–258.
- Kiyota, M.; Numayama, N.; Goto, K. Circadian rhythms of the L-ascorbic acid level in Euglena and spinach. J. Photochem. Photobiol. B Biol. 2006, 84, 197–203.
- Bolige, A.; Goto, K. High irradiance responses involving photoreversible multiple photoreceptors as related to photoperiodic induction of cell division in Euglena. J. Photochem. Photobiol. B Biol. 2007, 86, 109–120.
- Lebert, M.; Porst, M.; Häder, D.-P. Circadian rhythm of gravitaxis in Euglena gracilis. J. Plant Physiol. 1999, 155, 344–349.
- Nasir, A.; Strauch, S.; Becker, I.; Sperling, A.; Schuster, M.; Richter, P.; Weißkopf, M.; Ntefidou, M.; Daiker, V.; An, Y. The influence of microgravity on Euglena gracilis as studied on Shenzhou 8. Plant Biol. 2014, 16, 113–119.
- Häder, D.-P.; Lebert, M. Graviperception and gravitaxis in algae. Adv. Space Res. 2001, 27, 861–870.
- Richter, P.R.; Strauch, S.M.; Ntefidou, M.; Schuster, M.; Daiker, V.; Nasir, A.; Haag, F.W.M.; Lebert, M. Influence of different light-dark cycles on motility and photosynthesis of Euglena gracilis in closed bioreactors. Astrobiology 2014, 14, 848–858.
