Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Neurosciences
Autism is a neurodevelopmental condition that starts in childhood and continues into adulthood. The core characteristics include difficulties with social interaction and communication, together with restricted and repetitive behaviours. There are a number of key abnormalities of brain structure and function that trigger these behavioural patterns, including an imbalance of functional connectivity and synaptic transmission, neuronal death, gliosis and inflammation. In addition, autism has been linked to alterations in the gut microbiome.
- infrared
- non-pharmacological
- red
- cell death
1. Introduction
Autism spectrum disorder (referred henceforth to as “autism”) is characterised by two core symptoms; first, problems in social communication and interaction across contexts, and second, restricted, repetitive behaviours, interests, and activities [1]. It first becomes evident during early childhood, being about five times more prevalent in males than females, and follows into adulthood. Autism is clinically complex and the severity of symptoms varies widely between individuals given this diagnosis. It is associated frequently with various co-morbidities, including sensory and motor abnormalities, epilepsy, sleep disturbances, attention deficit and hyperactivity. Over recent years, the prevalence of autism has grown, with the current rate being approximately 1 in 160 [1,2,3,4,5,6,7,8,9].
2. The Mechanisms
The factors responsible for the brain changes leading to an expression of autism are not entirely clear, but there is a strong genetic basis, with approximately 90% concordance for monozygotic twins. There is considerable heterogeneity in the genetics however, with no one single genetic mutation accounting for more than 1–2% of all cases; further, there are rich interactions between multiple genes and the environment, making things even more complex. Maternal nutrition, autoimmune disease and inflammation, and/or exposure to air pollutants (e.g., heavy metals) or various drugs (e.g., thalidomide or valproic acid) during preconception and pregnancy can aggravate a genetic problem or damage the brain, increasing the risk of autism [2,3,5,9].
Some of the major abnormalities in brain structure and function associated with autism are outlined below (Figure 1A).
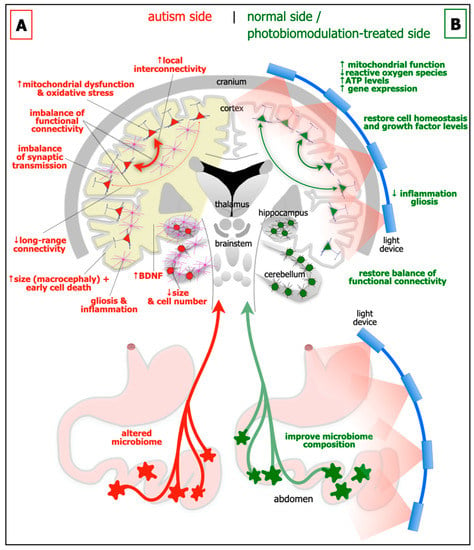
Figure 1. Schematic diagrams of the major abnormalities evident in autism (A; left side) as compared to normal, and after photobiomodulation treatment (B; right side). Autism is characterised by an altered microbiome in the gastrointestinal system (red star-like shapes), decrease in size of cerebellum and cerebellar cell number, increase in brain-derived neurotrophic factor (BDNF) levels in brain (yellow shade) and blood plasma, gliosis and inflammation in brain (pink cells), macrocephaly (increase in size of cortex), decrease in activity of long-range connectivity in cortex (thin red arrows), synaptic imbalance in brain, imbalance of functional connectivity, dysfunction and oxidative stress in brain (red cells) and increase in local interconnectivity in cortex (thick red arrows). We hypothesise that many if not all of these abnormalities will improve after photobiomodulation treatment to the head and to the abdomen (green cells and arrows). In particular, photobiomodulation will prompt; an increase in mitochondrial function, adenosine triphosphate (ATP) levels and gene expression, a reduction of oxidative stress, inflammation and gliosis, a restoration of cell homeostasis and growth factor levels, together with a restoration of a balanced functional activity across the brain.
Brain size and cytoarchitecture abnormalities: In approximately 20% of children with autism, there are general increases in the size of the cerebral cortex (ie brain overgrowth or macrocephaly), in particular the frontal, parietal and temporal areas. These early increases in size during childhood, appear to be followed by a premature decrease, presumably due to cell death (see below), from adolescence to late middle age [11,12]. But not all brain regions show the same patterns; the cerebellum for example, is generally smaller in children with autism (Figure 1A) [13,14]. Together with these abnormalities of size, there are distinct deficits in cytoarchitecture across different regions of the brain in autism. In the prefrontal cortex, particularly layer II, there are more neurones and fewer astrocytes, a feature linked to a developmental failure of radial glial cells to help immature neurones migrating to their appropriate cortical layer [15]. In the cerebellum, there are fewer cerebellar Purkinje and granule cells (Figure 1A) [13,14], while in the amygdala and hippocampus, there are differences in both size and in overall cell numbers [16,17].
Functional connectivity imbalance: There is an imbalance of functional connectivity across the brain in autism. These involve prefrontal, anterior cingulate, inferior parietal, and superior temporal cortices; these areas are associated with language, personality, task-switching, self-control, planning, working memory, social interactions and cognition, and many of the executive brain functions [18,19]. It has been suggested that autism can be characterised by an increased local interconnectivity but decreased long-range connectivity (Figure 1A) [20].
Synaptic imbalance: The balance of excitatory and inhibitory synaptic transmission is disrupted in autism (Figure 1A). A range of synaptic molecules and proteins become dysfunctional, such as those involved with cell adhesion [3]. There are decreased levels of glutamine and abnormal levels of glutamate evident in blood plasma [21], as well as many diverse glutamate receptors across the cortex [3]. There are reduced levels of glutamic acid decarboxylase, the rate-limiting enzyme in γ-aminobutyric acid (GABA) production, together with fewer GABA receptors [22]. A dysfunctional serotonergic system also contributes to the excitatory and inhibitory imbalance [3]; there are increased levels of serotonin in blood plasma and various genes encoding the serotonin neurotransmission are defective [23].
Gliosis and inflammation: There are clear signs of gliosis and inflammation in autism (Figure 1A). In both animal models and in people with autism, astrocytes and microglia-particularly in the hippocampus and the cerebellum-become reactive and release pro-inflammatory cytokines that exacerbate the inflammatory condition [9,24].
Mitochondrial dysfunction and oxidative stress: In autism, there is considerable mitochondrial dysfunction and oxidative stress, particularly in the cortex, hippocampus and cerebellum (Figure 1A). This results in increased levels of reactive oxygen species, an elevation of lipid peroxidation, abnormal calcium homeostasis and neurotransmitter imbalance, leading to dysfunctional neuronal activity and subsequent neuronal death [9,25].
Growth factors: A fascinating feature of autism is that there are elevated levels of growth factors in the brain, for example brain-derived neurotrophic factor (BDNF) in both the cortex and hippocampus (Figure 1A) [7], and in blood sera [26]. BDNF is a key molecule in maintaining cell homeostasis and function, and is associated with neuronal plasticity and growth. It has been suggested that elevated levels of BDNF generates synaptic dysfunction and is toxic to cells, leading to difficulties with executive function and behaviour [9,27,28]. Another view would be that the increase in BDNF in autism is a compensatory effect, in an attempt to repair the mitochondrial damage and cellular dysfunction, perhaps related to the increase in cell death during adolescence and middle age in autism (see above).
Microbiome: In addition to the changes evident in the brain, autism has also been linked to alterations in the gastrointestinal microbiome (Figure 1A) [8,9,29]. The microbiome is made up of microorganisms (i.e., bacteria, fungi, viruses, archaea, bacteriophages and protozoa) that reside, either transiently or permanently, within the gastrointestinal system. It has been described as the body’s additional or virtual organ, the key interface between food and the body. The microbiome has a number of critical functions, including: the digestion of food; increasing energy yields; contributing to nutrition; regulating sugar use and production and fat storage; together with influencing the integrity of the gut wall lining itself. Through its close relationship with the immune system and the large number of nerves that control the gut, the microbiome can have an enormous influence on many areas of health and well-being. Quite remarkably, during early development, the microbiome has been shown to influence brain networks and connectivity, particularly those related social interaction and behaviour; the key link in this relationship, namely the gut-brain axis, is through the far-reaching vagus nerve. In germ-free mice, those devoid of all microorganisms, there are alterations in protein and gene expression patterns across the brain, particularly within the hippocampus and amygdala. These changes are coupled with displays of abnormal social behaviours. If the microbiome composition is restored post-weaning in the mice, there is, rather strikingly, a reversal of these abnormalities. There are also indications that people with autism have altered microbiomes. For example, children with autism have been reported to have abnormal microbiome composition compared to controls; further, that autistic children often have gastrointestinal problems, with the severity related closely to the degree of behavioural disorder. The analysis of faecal matter from autistic children revealed a low relative abundance in a plethora of bacterial genera, including Barnesiella, Parabacteroids, Alistipes putredinis, B. caccae, Bacteroides intestinihominis, and the mucolytic bacterium Akkermansia muciniphila and Bifidobacterium spp. The consequential reduction in associated bacterial-derived genes encoding for key enzymes involved in the synthesis of GABA, melatonin and butyric acid, as well as possible alterations in gut mucosal barrier with pathological implications deriving from changes in gastrointestinal permeability, are all promising targets for novel treatment strategies [8,9,29].
This entry is adapted from the peer-reviewed paper 10.3390/neurolint14040071
This entry is offline, you can click here to edit this entry!
