Acute kidney injury (AKI) and chronic kidney disease (CKD) are highly prevalent in patients with cancer, and they are associated with an increased risk of all-cause mortality.
- acute kidney injury (AKI)
- kidney replacement therapy (KRT)
- thrombotic microangiopathy (TMA)
- hematopoietic stem cell transplant (HSCT)
- tumor lysis syndrome (TLS)
1. Introduction
The last two decades have seen an exponential rise in the number of drugs used in cancer therapy. With precision medicine, novel targeted therapies, and better supportive care, the life expectancy of patients with cancer has improved [1]. Unfortunately, acute kidney injury (AKI) in the context of cancer has been increasingly recognized. Overall, AKI in cancer patients can be broadly classified as pre-renal or hemodynamic, intrinsic, and obstructive nephropathy. Nevertheless, malignancy adds a layer of complexity since AKI may be a direct complication of cancer itself (infiltration, paraneoplastic syndrome), cancer-related metabolic disturbances (hypercalcemia, tumor-lysis syndrome), anti-cancer therapy (chemotherapy, immune checkpoint inhibitors, stem-cell transplant) or other related complications (hypovolemia, infections, sepsis) (Figure 1).
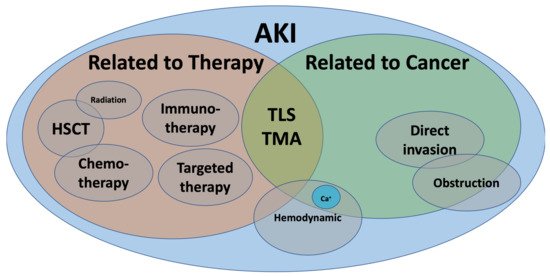
Figure 1. Acute kidney injury in cancer.AKI: acute kidney injury, Ca+: calcium, HSCT: hematopoietic stem cell transplant, TLS: tumor lysis syndrome, TMA: thrombotic microangiopathy.
2. Epidemiology of AKI in Patients with Cancer
Several different studies have defined the incidence of AKI related to cancer. Christiansen et al. described the incidence of AKI in all incident cancer patients in a population-based study in Denmark (n = 44, 116). The risk of developing AKI criteria was 17.5% during the first year and up to 27% during the first five years of cancer diagnosis [1]. A population-based study from Ontario, Canada, reported a cumulative incidence of AKI of 9.3% [2]. Similarly, a population-based study from China reported an incidence of AKI in patients with cancer of 7.5% [3]. The malignancies most frequently associated with AKI are multiple myeloma, kidney, liver, bladder and lymphoma and leukemia [4,5,6,7,8,9]. The risk factors for developing AKI are cancer stage, previous chronic kidney disease (CKD), diabetes mellitus and use of angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs) [10]. Moreover, AKI in hospitalized patients has been linked to an increased length of hospital stay and costs of care [5,6].
3. Acute Kidney Injury in Critically Ill Patients with Cancer
Acute kidney injury occurs in 50–60% of patients admitted to the intensive care unit, and 20% of those patients have an underlying malignancy [7]. The frequency of AKI and kidney replacement therapy (KRT) has increased over the past couple of decades, perhaps due to better survival and a higher admission rate of patients with cancer to the intensive care units [1,8,9]. Critically ill patients with cancer are exceptionally susceptible to AKI, and the incidence of requiring KRT varies from 8–13% in patients with solid tumors and 10–34% in patients with hematological malignancies [10]. The risk of developing AKI is more significant in patients with septic shock, exposure to nephrotoxins, obstructive nephropathy and hematological malignancies, especially multiple myeloma [3,10,11,12] The short-term mortality of critically ill patients with cancer and severe AKI is comparable to critically ill patients without cancer [13,14]. In a cohort of critically ill patients with cancer (n = 975), the in-hospital and six-month mortality of AKI requiring KRT was 64% and 73%, respectively [12]. The need for KRT entails high mortality, and for patients who are not dialysis candidates, palliative care consult services can be helpful.
4. Hemodynamic Causes of Acute Kidney Injury in Patients with Cancer
Patients with cancer are susceptible to a myriad of hemodynamic insults. Oncology patients experience anorexia, nausea and vomiting in 60–80% of the cases [15]. A careful physical examination looking for signs of volume contraction should always be performed. Nonetheless, the sensitivity and specificity of physical examination are low. Initial investigations should include serum electrolytes, urea or blood urea nitrogen, and creatinine levels. A point of care ultrasound can further inform our evaluation in differentiating hypovolemia from other causes of AKI [16,17,18].
Hypercalcemia complicates up to 30% of all malignancies and causes AKI by several mechanisms [19]. Hypercalcemia leads to severe volume depletion via the activation of the calcium sensor located in the thick ascending loop of Henle, causing a furosemide-like effect [20]. Hypercalcemia leads to the vasoconstriction of the afferent arteriole, decreasing intra-glomerular pressure [21]. Finally, the precipitation of calcium phosphate crystals and clogging of the tubules have been described [22]. The initial treatment should be directed to restore intravascular volume with crystalloids (intravenous normal saline 200–250 mL/Hr). Loop diuretics are reserved for patients with volume overload. Anuric patients may become fluid overloaded rapidly and be unresponsive to diuretics; hence hemodialysis with low calcium baths should be performed in these cases. Calcitonin or bisphosphonates are indicated after the initial resuscitation [23]. The preferred bisphosphonates are pamidronate and ibandronate; however, zoledronic acid may be superior and has been used successfully in patients with serum Cr <4.5 mg/dL with a similar safety profile [24]. Zoledronic acid is not recommended for chronic use in patients with an estimated glomerular filtration rate of eGFR <30 mL/min/m2 [25]. Denosumab, a neutralizing monoclonal antibody directed against the receptor activator of nuclear factor kb ligand, has been used for the treatment of cancer-related hypercalcemia [26,27].
Contrast-associated acute kidney injury (CA-AKI) has been often cited as a common cause of AKI [28]. However, the relevance and causal relationship between contrast medium and intrinsic AKI have been questioned [29]. The use of iso-osmolar and low-osmolar contrast agents in low quantities has improved these procedures’ safety [30]. The rise in serum creatinine associated with contrast may be explained by intraglomerular hemodynamic changes rather than intrinsic tubular damage [31,32]. In patients with an estimated glomerular filtration rate (eGFR) of >45 mL/min/1.73 m2, the risk of AKI is negligible. Caution is advised in patients with a lower eGFR who also have other risk factors for CA-AKI. For these patients, prophylactic IV fluids are advised [33].
Heart failure (HF) is another hemodynamic derangement commonly encountered in patients with cancer. Besides the common causes of HF, it is essential to inquire about previous exposure to cardiotoxic chemotherapy such as anthracyclines (doxorubicin, daunorubicin, and epirubicin) and the human epidermal growth factor receptor 2 (HER2) modulator trastuzumab [34]. Type 1 cardiorenal syndrome is the result of a decreased eGFR secondary to kidney hypoperfusion. Low cardiac output and intra-renal venous congestion are the main drivers of this pathological condition. Maladaptive neurohormonal changes such as the upregulation of the renin–angiotensin–aldosterone system (RAAS), the non-osmotic release of vasopressin and the sympathetic nervous system’s activation result in increased sodium and water reabsorption [35,36]. Decongestion with a loop diuretic alone or in combination with other classes of diuretics are the first step in treating this pathology [37].
Liver injury or cirrhosis are associated with hepatorenal syndrome (HRS), a diagnosis of exclusion. Portal hypertension causes nitric oxide-mediated splanchnic vasodilation, with secondary pooling of blood in the splanchnic circulation and hypotension. Similarly, the activation of RAAS and other neurohumoral systems leads to kidney vasoconstriction, hypoperfusion, and the retention of salt and water. Management includes treating HRS precipitants and restoring effective arterial circulation with vasopressors: terlipressin, norepinephrine or midodrine in combination with octreotide [38].
Tense ascites may increase intraabdominal pressure, causing abdominal compartmental syndrome. Paracentesis with albumin replacement may improve kidney hemodynamics and help alleviate AKI [39]. The sinusoidal obstruction syndrome, a complication of stem-cell transplant, is similar to hepatorenal syndrome due to the associated portal hypertension secondary to the hepatic sinusoidal injury.
5. Cancer-Associated Thrombotic Microangiopathy (TMA)
Cancer-associated thrombotic microangiopathy (TMA) refers to a constellation of disorders characterized by microvascular thrombosis, thrombocytopenia, and resultant ischemia of the end organ affected, e.g., kidney and brain [40]. The pathological characteristics of TMA include intrarenal or systemic microvascular thrombosis with endothelial swelling and microvascular obstruction (Figure 2) [41].
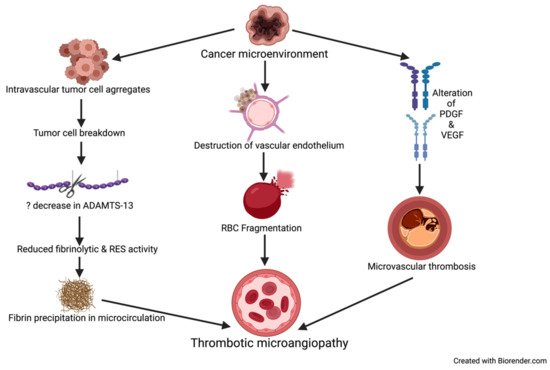
Figure 2. Mechanisms causing TMA in patients with cancer. ADAMTS-13 = a disintegrin and metalloproteinase with thrombospondin type-1 motif, member 13, PDGF = platelet derived growth factor. VEGF = vascular endothelial growth factor, RBC = red blood cell, RES = reticuloendothelial system.
TMA syndromes are a complication of cancer itself and can also occur as a side effect of cancer chemotherapeutic agents [42]. One of the earliest reported studies on TMA in cancer patients is from 1972 from Germany, which showed 5.7% of patients with metastatic cancer have TMA [43]. Gastric carcinoma tops the list (50%), followed by breast and lung carcinoma (Table 1).
Table 1. Cancers associated with TMA [44,45,46,47,48,49].
|
System. |
Cancers. |
|---|---|
|
Gastrointestinal Tract |
Gastric Cancer Colon Cancer Carcinoma of the Anal Canal (Squamous Cell Carcinoma) Metastatic Appendiceal Carcinoma |
|
Lung |
Adenocarcinoma Squamous Cell Carcinoma Small Cell Lung Cancer |
|
Genitourinary Tract |
Prostate Cancer Ovarian Cancer Renal Cell Carcinoma Seminal Vesicle Tumor. |
|
Hepatobiliary System |
Hepatocellular Carcinoma Pancreatic Cancer Cholangiocarcinoma |
|
Endocrine System |
Multiple Endocrine Neoplasia Type 1 Pheochromocytoma Neuroendocrine Tumor Prolactin-Producing Pituitary Adenoma |
|
Hematologic Malignancies |
Non-Hodgkin Lymphoma Acute Lymphoblastic Leukemia Myelodysplastic Syndrome Hodgkin Lymphoma Multiple Myeloma |
|
Others |
Breast Cancer Kaposi Sarcoma Carcinoma of Unknown Origin |
6. Acute Kidney Injury Due to Renal Parenchymal Invasion/Infiltrative Malignancies
Many solid and hematological cancers may involve the renal parenchyma. Lymphomas and leukemias are the most common cancers that demonstrate autopsy evidence of infiltration, with the incidence being 6% to 60% [57,58]. Lymphomatous invasion of the kidneys (LIK) can present as acute kidney injury, new-onset or worsening proteinuria and hematuria; however, diagnosis is usually incidental. In approximately one percent of cases, the tumor burden infiltrating the kidneys can be so high that it can lead to AKI [59,60]. Tornroth et al. demonstrated various pathological phenotypes of lymphomatous invasion of kidneys [61]. Most cases (87%) showed interstitial infiltration followed by intraglomerular infiltration (45%). Renal imaging in these cases shows bulky and enlarged kidneys. A high index of suspicion is necessary to prompt a kidney biopsy. Another case series showed that 34% of non-Hodgkin’s lymphoma developed parenchymal kidney invasion; however, only 14% were diagnosed before death [62]. For indolent hematological cancers such as chronic lymphocytic leukemia, which are often not treated unless there is end-organ involvement, the demonstration of LIK may often pull the trigger to initiate chemotherapy. The most common solid organ cancers metastasizing to the kidneys are lung carcinoma, gastric, breast and malignant melanoma [11]. Renal metastases usually manifest as bilateral, small, multifocal parenchymal nodules, though single exophytic lesions have also been described [63]. Metastases to kidneys are seen in a setting of massive tumor burden and portend a poor prognosis. Acute kidney injury from infiltrative cancers results from renal parenchymal compression, which leads to the disruption of the glomerular, tubulointerstitial and microvascular architecture, leading to impairment of the GFR. Most cases are subclinical; however, patients may present with hypertension (the upregulation of the renin–angiotensin axis), flank pain (due to stretching of renal capsule) and hematuria.
7. Hematopoietic Stem Cell Transplant-Related Acute Kidney Injury
Acute kidney injury after hematopoietic stem cell transplant (HSCT) is usually defined as a doubling of baseline serum creatinine or decline in GFR of at least 50% within the first 100 days after engraftment [64]. It was difficult to determine the epidemiology of HSCT-related AKI due to inconsistencies in the AKI definition. Hence, an attempt was made to develop uniformity to gain insight into the epidemiology of AKI and facilitate the comparison of studies. Recent studies have used criteria such as the risk, injury, failure, loss of kidney function, end-stage kidney disease (RIFLE) system and the Acute Kidney Injury Network (AKIN) criteria for kidney injury. A doubling of the serum creatinine level is correlated with RIFLE-I (injury to the kidney) and AKIN stage 2 [64].
AKI occurs in 12–21% of patients undergoing autologous HSCT and majorly depends on the type of conditioning used after allogenic HSCT. Myeloablative conditioning and reduced-intensity conditioning (RIC) are associated with 35–56% and 7–46% incidence of AKI [64,65,66,67,68,69]. Kidney replacement therapy requirement in autologous HSCT, myeloablative allogenic HSCT and RIC is 7%, 20–33% and 4%, respectively [70]. Not only AKI occurring within the first 30 days of HSCT, but also its severity portends an an increased risk of death and overall low survival rates [64,71,72]. Among patients who require KRT, the mortality is exceedingly high (55–100%) [73,74].
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics11040611
