Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Neonates do experience pain and its management is necessary in order to prevent long-term, as well as, short-term effects. The most common source of pain in the neonatal intensive care unit (NICU) is caused by medically invasive procedures. Non-pharmacological interventions, particularly breastfeeding and non-nutritive sucking as primary strategies for pain management in neonates are useful strategies to consider.
- NICU
- breast feeding
- pain management
- non-nutritive sucking
- oral sucrose
1. Introduction
Contrarily to past practices and beliefs, newborns can indeed feel and understand pain stimulus, with premature infants been even more susceptible to pain [1]. Heavy pain for the newborn can affect physiologically all the main organ systems with potential severe consequences in later on [1]. Neonatal nervous systems are in continuous development, thus, they are prone to neurodevelopmental changes from painful stimuli [2], with effects evident even in adult life [3][4]. Even more so for premature neonates that are put into neonatal intensive care units (NICU) in order to become fully grown infants. Their requirements for stimuli and nutrients are at the foetus level and need to be highly regulated to reach the appropriate level of development [5]. NICU has seen many advance in the past years but, accommodation in one does not secure the required development especially for very low birth weight (<1500 g) infants [6][7]. Stunted growth is directly linked to neurodevelopmental problems [8][9] that in turn are linked to worse sensory management for preterm neonates, making them more sensitive to pain [10].
Nutritional practices in NICU are increasingly incorporate breast feeding in their regimes considering it a medical intervention for the correct development of the neonate [11][12][13][14]. Human milk is not the only milk supplied nor breast feeding the only delivery method. Cow’s milk and enriched milk products are also utilized but human milk has many health inductive properties that make it more that nutritional and indeed more medicinal in nature [15][16][17][18]. Breastfeeding releases antioxidant and anti-inflammatory substances to the infant [19], helps establish the gut microbiome and the immune system [20][21] while promoting neurological development [22]. An additional and equally important reason is the required parental presence enhancing the parental-infant interaction that is also an indicator for a succesful outcome after NICU [18].
2. Non-Pharmacological Methods
Non-pharmacological methods, such as non-nutritive sucking [23], skin-to-skin care [24][25], swaddling/facilitated tucking [26], rocking/holding [25][27], and music [28][29] have been recently found to be effective as pain relief strategies in infants in the NICU. In addition, Shah, et al. (2012) found administering glucose/sucrose offered similar pain relief to breastfeeding in neonates [28].
Table 1 summarizes all the common pain management methods in NICU.
Table 1. Most common pain management methods in NICU.
| Pharmacological Methods | Type of Pain Management |
|---|---|
| Analgesics | |
| Paracetamol [29] | Mild to moderate pain |
| Opioids, mostly Morphine & Fentanyl [30] | Persistent pain |
| Methadone [31], ketamine, propofol, dexmedetomidine [32]. | Persistent pain, limited use |
| Non-Steroidal Anti-Inflammatory Drugs [33] | Not recommended for infants < 6 months of age, due to established adverse side effects |
| Sedatives | |
| Benzodiazepines- Midazolam, [34] | Sedation |
| Non-pharmacological methods | Type of Pain Management |
| Non-nutritive sucking [23]. Provision of a pacifier or the sucking of the fingers or the hand during painful event |
Acute procedural Mild to moderate pain |
| Skin-to-skin care (kangaroo care) [24][25]. Newborns wearing only a diaper being held next to their mother’s bare chest |
Acute procedural Mild to moderate pain |
| Swaddling/Facilitated tucking [26] Wraping the infant tightly/Holding the infant in the side-lying, flexed fetal-type position by hand |
Acute procedural Mild pain |
| Rocking/holding [25][27]. Holding the neonate and swaying in an rocking motion |
Acute procedural Mild pain |
| Music listening [35], Recorded maternal singing [36] White noise/classical music playing during painfull procedures [37] |
Pasification, Recovery reinforcement of sucking, Acute procedural pain and stress relief |
| Breastfeeding for a duration of more than 2 min prior to a painful procedure [28][38][39][40][41][42][43][44][45][46][47] |
Acute procedural pain mild to medium |
| Oral administration of Sucrose/glucose [48][49][50] | Acute procedural pain mild to medium, short lived duration |
2.1. Non-Nutrient Sucking
Non-nutritive sucking (NNS), is the provision of a pacifier or the sucking of the fingers or the hand in neonates of NICU. NNS is considered a safe and effective method of pain relief during the pinprick procedure in neonates [23]. But it is more effective in conjunction with sucrose/glucose sucking [48]. A randomized controlled clinical trial with cross-over design [51] in an Iranian NICU with 60 infants demonstrated the effectiveness of using oral dextrose for pain management during a heel prick in comparison to facilitated tucking. They did find also that facilitated tucking is effective compared to no management and can be utilized in constrained situations or in combination with oral dextrose [51]. According to a recent study [52], where sucrose was compared with non-nutritive suction, it appeared that with the help of the NIPS scale, sucrose was superior in reducing the duration of crying when removing adhesive patches from newborn wounds. But it was noted that sucrose alone is not superior to behavioral pain management compared to the combination of methods [52]. A significant reduction in pain scores was found in neonates with NNS as well as sucrose administration compared to NNS or sucrose alone in a 2022 study [48] albeit for mild pain caused. Short duration of the sucrose administration pain relief effect has been reported previously [49][50]. NNS usage in the contex of Point of care quality improvement method (POCQI) using a commercially fixed dosage oral sucrose solution gave a 96% rise in NNS use, in a level 3 NICU in India [53].
2.2. Breastfeeding
Breastfeeding in NICU has to be initiated and then established for the neonates to automate the process by tube and then progress to oral feeding after they are developed enough [11][54][55]. When established bottle-feeding and breastfeeding are the most common delivery methods for maternal milk even though exclusive breastfeeding is the gold standard recommended for at least first six months [56][57].
Breastfeeding utilization in the NICU becomes highly important, as studies are published daily with the properties and benefits of breast milk [58][59]. Newborns undoubtedly need their mother’s contact and proper nutrition for their future development. Breastfeeding for a duration of more than 2 min prior to a painful procedure [39] is a valid non pharmacological pain management method. While, the presence of the mother to breastfeed, especially in very premature infants is not applicable, it is a valid option in more grown infants [39].
Direct-breastfeeding is the direct suckling on the breast regardless of the delivery of milk to the infant [60], while expressed breast feeding is the extraction and storage of milk for later delivery with a bottle [61]. Direct breastfeeding is the unequivocal best practice in non-pharmacological pain management methods since it has been compared to all other methods and has been found more effective [38]. It fared much better compared to swaddling [28][40], maternal holding [41] or skin-to-skin care [42][43], topical anesthetics [44] and cooling sprays [45], non-nutritive sucking [46] and music [47] in pain management.
A well-known Cochrane systematic review and meta-analysis [28] from 20 studies (1075 direct and 996 expressed breast feeding infants) established the pain management effectiveness of breastfeeding either direct or in full-term infants. A later systematic review [62] for 15 studies with 1908 infants in total, was more explicit in their results.
Direct breast-feeding was stated as the best method of non-pharmacological pain management compared to all other (holding, skin-to-skin contact, topical anesthetics, and music), and was preferable even to administration of glucose/sucrose in full-term infants [28]. While they did not recommend expressed breast milk as they deemed it not effective enough for pain relief [28].
2.3. Non-Pharmacological Methods Used by Parents
Despite the effectiveness of non-pharmacological methods for procedural pain management in infants, being evident [63], researchers know precious little about the actual methods the NICU infant parents use. Campbell-Yeo et al. (2011) believed that such strategies are mostly used by nurses to hold on to NICU authority over infant caregiving, despite parents wishing to be more engaged in comforting their infants [39]. Parental involvement in infant pain management in NICU has been previously addressed and needs to be higher [64][65].
A unique cross-sectional and descriptive study of 178 parents whose newborns were placed in NICUs in Finland [66] found that most parents almost exclusively used physical methods, such as touching, holding, and positioning. Very few used other established NICU strategies such as breastfeeding, with only 2% of the parents utilizing it and NNS with oral sucrose (6%). They stated that parental pain management was relate to newborn condition and gestational age [66]. Parents did not use many valid strategies, such as swaddling, facilitated tucking/kangaroo care, music, breastfeeding, and NNS/sucrose [66]. Parents used easy to copy and perform methods that did not require to be taught by the nursing staff, clearly lacking training and knowledge on these effective, yet more difficult to master, strategies [66]. They concluded with a plea to extend parents’ use and knowledge of non-pharmacological pain management methods to manage their infants’ procedural pain in the NICU [66].
The majority of non-pharmacological pain management methods are more effective performed by the parents rather than NICU staff [39]. Thus, NICU staff and healthcare professionals must enable parents to follow such methods, by providing guidance and training for a more active involvement into their child’s care while in NICU [67][68].
3. Pharmacological Methods
Historically, several accepted pain management methods over the years have changed due to undesirable clinical results [30]. It is generally accepted that the use of pharmacological methods in NICU is a controversial issue, as the goal of the medical and nursing staff is not only to deal with short term pain, but also to properly manage the incident, in order to mitigate the subsequent consequences in the long term [69]. In addition, it is worth noting that most non-steroidal anti-inflammatory drugs (NSAIDs) are not recommended for infants < 6 months of age, due to established adverse side effects [33]. The most widely used NSAID is paracetamol [29] for mild to moderate pain relief, and to reduces the need to use morphine [70], thus reducing the risk of opioid addiction. Intubation and mechanical ventilation are usually the procedures that opioids such as fentanyl and morphine are used for, since they are causes for persistent pain [30]. Recent research is inconclusive whether opioids have an effect on pain and neurodevelopmental outcomes at later age [71][72]. Also morphine or fentanyl usage probably has limited effectiveness on reducing the duration of mechanical ventilation and neonatal mortality [73].
Midazolam, and its family of substances benzodiazepines are in use in NICU especially for sedation. As they are found to strengthen opioid effectiveness in causing respiratory depression and hypotension safety concern have been raised [34]. Several other substances some controlled, methadone [31], ketamine, propofol, and dexmedetomidine, where considered, but very limited data and known side effects have restricted their use [32].
In a recent study it was shown that the use of morphine allowed enhanced pain relief compared to its combination with midazolam in NICU, with a lower cost. Thus, morphine alone stands as a common analgesia strategy especially in neonates with respiratory distress syndrome (RDS) [74]. Figure 1 summarizes the main findings.
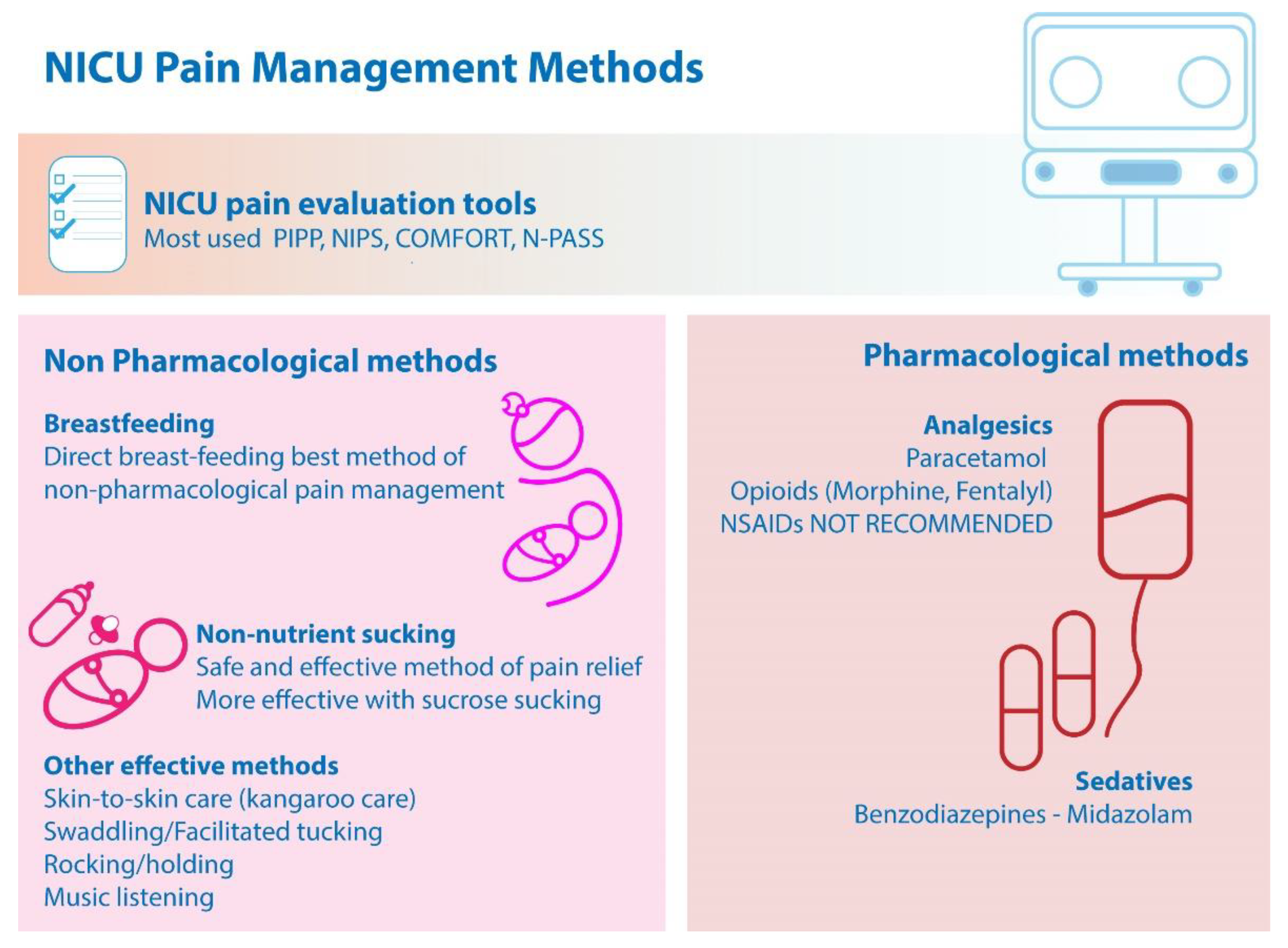
Figure 1. Main findings on NICU pain management methods.
This entry is adapted from the peer-reviewed paper 10.3390/children9101568
References
- Perry, M.; Tan, Z.; Chen, J.; Weidig, T.; Xu, W.; Cong, X.S. Neonatal pain: Perceptions and current practice. Crit. Care Nurs. Clin. 2018, 30, 549–561.
- Williams, M.D.; Lascelles, B.D.X. Early neonatal pain—A review of clinical and experimental implications on painful conditions later in life. Front. Pediatr. 2020, 8, 30.
- Grunau, R.E.; Holsti, L.; Peters, J.W. Long-term consequences of pain in human neonates. Semin. Fetal Neonatal Med. 2006, 11, 268–275.
- Walker, S.; Melbourne, A.; O’Reilly, H.; Beckmann, J.; Eaton-Rosen, Z.; Ourselin, S.; Marlow, N. Somatosensory function and pain in extremely preterm young adults from the UK EPICure cohort: Sex-dependent differences and impact of neonatal surgery. Br. J. Anaesth. 2018, 121, 623–635.
- Agostoni, C.; Buonocore, G.; Carnielli, V.; De Curtis, M.; Darmaun, D.; Decsi, T.; Domellöf, M.; Embleton, N.D.; Fusch, C.; Genzel-Boroviczeny, O. Enteral nutrient supply for preterm infants: Commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 85–91.
- Horbar, J.D.; Ehrenkranz, R.A.; Badger, G.J.; Edwards, E.M.; Morrow, K.A.; Soll, R.F.; Buzas, J.S.; Bertino, E.; Gagliardi, L.; Bellù, R. Weight growth velocity and postnatal growth failure in infants 501 to 1500 grams: 2000–2013. Pediatrics 2015, 136, e84–e92.
- Ramel, S.E.; Demerath, E.W.; Gray, H.L.; Younge, N.; Boys, C.; Georgieff, M.K. The relationship of poor linear growth velocity with neonatal illness and two-year neurodevelopment in preterm infants. Neonatology 2012, 102, 19–24.
- Ehrenkranz, R.A.; Dusick, A.M.; Vohr, B.R.; Wright, L.L.; Wrage, L.A.; Poole, W.K.; Health, N.I.o.C.; Network, H.D.N.R. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 2006, 117, 1253–1261.
- Sammallahti, S.; Kajantie, E.; Matinolli, H.-M.; Pyhälä, R.; Lahti, J.; Heinonen, K.; Lahti, M.; Pesonen, A.-K.; Eriksson, J.G.; Hovi, P. Nutrition after preterm birth and adult neurocognitive outcomes. PLoS ONE 2017, 12, e0185632.
- Grunau, R.E. Neonatal pain in very preterm infants: Long-term effects on brain, neurodevelopment and pain reactivity. Rambam Maimonides Med. J. 2013, 4, e0025.
- Briere, C.E.; McGrath, J.; Cong, X.; Cusson, R. An integrative review of factors that influence breastfeeding duration for premature infants after NICU hospitalization. J. Obstet. Gynecol. Neonatal Nurs. 2014, 43, 272–281.
- Bujold, M.; Feeley, N.; Axelin, A.; Cinquino, C.; Dowling, D.; Thibeau, S. Expressing human milk in the NICU. Adv. Neonatal Care 2018, 18, 38–48.
- Parker, M.G.; Stellwagen, L.M.; Noble, L.; Kim, J.H.; Poindexter, B.B.; Puopolo, K.M. Promoting human milk and breastfeeding for the very low birth weight infant. Pediatrics 2021, 148, e2021054272.
- Lavallée, A.; De Clifford-Faugère, G.; Garcia, C.; Oviedo, A.N.F.; Héon, M.; Aita, M. PART 2: Practice and research recommendations for quality developmental care in the NICU. J. Neonatal Nurs. 2019, 25, 160–165.
- Chetta, K.E.; Schulz, E.V.; Wagner, C.L. Outcomes improved with human milk intake in preterm and full-term infants. Semin. Perinatol. 2021, 45, 151384.
- Ottolini, K.M.; Andescavage, N.; Keller, S.; Limperopoulos, C. Nutrition and the developing brain: The road to optimizing early neurodevelopment: A systematic review. Pediatr. Res. 2020, 87, 194–201.
- Hortensius, L.M.; Janson, E.; van Beek, P.E.; Groenendaal, F.; Claessens, N.H.; Swanenburg de Veye, H.F.; Eijsermans, M.J.; Koopman-Esseboom, C.; Dudink, J.; van Elburg, R.M. Nutritional intake, white matter integrity, and neurodevelopment in extremely preterm born infants. Nutrients 2021, 13, 3409.
- Belfort, M.B.; Inder, T.E. Human milk and preterm infant brain development: A narrative review. Clin. Ther. 2022, 44, 612–621.
- Choudhury, V.; Amin, S.B.; Agarwal, A.; Srivastava, L.; Soni, A.; Saluja, S. Latent iron deficiency at birth influences auditory neural maturation in late preterm and term infants. Am. J. Clin. Nutr. 2015, 102, 1030–1034.
- Andreas, N.J.; Kampmann, B.; Mehring Le-Doare, K. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635.
- Paramasivam, K.; Michie, C.; Opara, E.; Jewell, A.P. Human breast milk immunology: A review. Int. J. Fertil. Women’s Med. 2006, 51, 208–217.
- Li, R.; Xia, W.; Zhang, Z.; Wu, K. S100B protein, brain-derived neurotrophic factor, and glial cell line-derived neurotrophic factor in human milk. PLoS ONE 2011, 6, e21663.
- Vu-Ngoc, H.; Uyen, N.C.M.; Thinh, O.P.; Don, L.D.; Danh, N.V.T.; Truc, N.T.T.; Vi, V.T.; Vuong, N.L.; Huy, N.T.; Duong, P.D.T. Analgesic effect of non-nutritive sucking in term neonates: A randomized controlled trial. Pediatr. Neonatol. 2020, 61, 106–113.
- Ludington-Hoe, S.M.; Johnson, M.W.; Morgan, K.; Lewis, T.; Gutman, J.; Wilson, P.D.; Scher, M.S. Neurophysiologic assessment of neonatal sleep organization: Preliminary results of a randomized, controlled trial of skin contact with preterm infants. Pediatrics 2006, 117, e909–e923.
- Pillai Riddell, R.; Racine, N.; Turcotte, K.; Uman, L.; Horton, R.; Ahola Kohut, S.; Din Osmun, L.; Hillgrove-Stuart, J.; Stevens, B.; Lisi, D. Non-pharmacological management of infant and young child procedural pain: An abridged Cochrane review. Cochrane Database Syst. Rev. 2011, 10, CD006275.
- Axelin, A.; Salanterä, S.; Lehtonen, L. ‘Facilitated tucking by parents’ in pain management of preterm infants—A randomized crossover trial. Early Hum. Dev. 2006, 82, 241–247.
- Chidambaram, A.G.; Manjula, S.; Adhisivam, B.; Vishnu Bhat, B. Effect of Kangaroo mother care in reducing pain due to heel prick among preterm neonates: A crossover trial. J. Matern. Fetal Neonatal Med. 2014, 27, 488–490.
- Shah, P.S.; Herbozo, C.; Aliwalas, L.L.; Shah, V.S. Breastfeeding or breast milk for procedural pain in neonates. Cochrane Database Syst. Rev. 2012.
- Pacifici, G.M.; Allegaert, K. Clinical Pharmacology of Paracetamol in Neonates: A Review. Curr. Ther. Res. 2015, 77, 24–30.
- Keels, E.; Sethna, N.; Watterberg, K.L.; Cummings, J.J.; Benitz, W.E.; Eichenwald, E.C.; Poindexter, B.B.; Stewart, D.L.; Aucott, S.W.; Goldsmith, J.P. Prevention and management of procedural pain in the neonate: An update. Pediatrics 2016, 137, 1–13.
- Anand, K.J. Pharmacological approaches to the management of pain in the neonatal intensive care unit. J. Perinatol. 2007, 27 (Suppl. S1), S4–S11.
- Durrmeyer, X.; Vutskits, L.; Anand, K.J.; Rimensberger, P.C. Use of analgesic and sedative drugs in the NICU: Integrating clinical trials and laboratory data. Pediatr. Res. 2010, 67, 117–127.
- Eccleston, C.; Cooper, T.E.; Fisher, E.; Anderson, B.; Wilkinson, N.M. Non-steroidal anti-inflammatory drugs (NSAIDs) for chronic non-cancer pain in children and adolescents. Cochrane Database Syst. Rev. 2017, 8, Cd012537.
- Ng, E.; Taddio, A.; Ohlsson, A. Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit. Cochrane Database Syst. Rev. 2017, 1, CD002052.
- Bergomi, P.; Chieppi, M.; Maini, A.; Mugnos, T.; Spotti, D.; Tzialla, C.; Scudeller, L. Nonpharmacological techniques to reduce pain in preterm infants who receive heel-lance procedure: A randomized controlled trial. Res. Theory Nurs. Pract. 2014, 28, 335–348.
- Corrigan, M.J.; Keeler, J.R.; Miller, H.D.; Ben Khallouq, B.A.; Fowler, S.B. Music therapy and retinopathy of prematurity screening: Using recorded maternal singing and heartbeat for post exam recovery. J. Perinatol. 2020, 40, 1780–1788.
- Dur, Ş.; Çevik, S.G.; Ustabaş Yıldız, N. The effect of white noise and classical music on pain and physiologic parameters in preterm infants during retinopathy of prematurity examinations: A randomized controlled trial. Early Child Dev. Care 2022, 1–12.
- Campbell-Yeo, M.; Eriksson, M.; Benoit, B. Assessment and Management of Pain in Preterm Infants: A Practice Update. Children 2022, 9, 244.
- Campbell-Yeo, M.; Fernandes, A.; Johnston, C. Procedural pain management for neonates using nonpharmacological strategies: Part 2: Mother-driven interventions. Adv. Neonatal Care 2011, 11, 312–318.
- Yilmaz, D.; Inal, S. Effects of three different methods used during heel lance procedures on pain level in term neonates. Jpn. J. Nurs. Sci. 2020, 17, e12338.
- Obeidat, H.M.; Shuriquie, M.A. Effect of Breast-Feeding and Maternal Holding in Relieving Painful Responses in Full-Term Neonates: A Randomized Clinical Trial. J. Perinat. Neonatal Nurs. 2015, 29, 248–254.
- Fallah, R.; Naserzadeh, N.; Ferdosian, F.; Binesh, F. Comparison of effect of kangaroo mother care, breastfeeding and swaddling on Bacillus Calmette-Guerin vaccination pain score in healthy term neonates by a clinical trial. J. Matern. Neonatal Med. 2017, 30, 1147–1150.
- Marín Gabriel, M.; del Rey Hurtado de Mendoza, B.; Jiménez Figueroa, L.; Medina, V.; Iglesias Fernández, B.; Vázquez Rodríguez, M.; Escudero Huedo, V.; Medina Malagón, L. Analgesia with breastfeeding in addition to skin-to-skin contact during heel prick. Arch. Dis. Child. Fetal Neonatal Ed. 2013, 98, F499–F503.
- Gupta, N.K.; Upadhyay, A.; Agarwal, A.; Goswami, G.; Kumar, J.; Sreenivas, V. Randomized controlled trial of topical EMLA and breastfeeding for reducing pain during wDPT vaccination. Eur. J. Pediatr. 2013, 172, 1527–1533.
- Boroumandfar, K.; Khodaei, F.; Abdeyazdan, Z.; Maroufi, M. Comparison of vaccination-related pain in infants who receive vapocoolant spray and breastfeeding during injection. Iran. J. Nurs. Midwifery Res. 2013, 18, 33–37.
- Lima, A.H.; Hermont, A.P.; Friche, A.A. Analgesia in newborns: A case-control study of the efficacy of nutritive and non-nutritive sucking stimuli. Codas 2013, 25, 365–368.
- Zhu, J.; Hong-Gu, H.; Zhou, X.; Wei, H.; Gao, Y.; Ye, B.; Liu, Z.; Chan, S.W. Pain relief effect of breast feeding and music therapy during heel lance for healthy-term neonates in China: A randomized controlled trial. Midwifery 2015, 31, 365–372.
- Li, Q.; Tan, X.; Li, X.; Tang, W.; Mei, L.; Cheng, G.; Zou, Y. Efficacy and safety of combined oral sucrose and nonnutritive sucking in pain management for infants: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0268033.
- Stevens, B.; Yamada, J.; Ohlsson, A.; Haliburton, S.; Shorkey, A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst. Rev. 2016, 7, Cd001069.
- Gibbins, S.; Stevens, B. The influence of gestational age on the efficacy and short-term safety of sucrose for procedural pain relief. Adv. Neonatal Care Off. J. Natl. Assoc. Neonatal Nurses 2003, 3, 241–249.
- Ranjbar, A.; Bernstein, C.; Shariat, M.; Ranjbar, H. Comparison of facilitated tucking and oral dextrose in reducing the pain of heel stick in preterm infants: A randomized clinical trial. BMC Pediatr. 2020, 20, 162.
- Mandee, S.; Buachai, K.; Aroonpruksakul, N.; Tantemsapya, N.; Buasuk, T. Effects of Sucrose and Nonnutritive Sucking on Pain Behavior in Neonates and Infants undergoing Wound Dressing after Surgery: A Randomized Controlled Trial. Eur. J. Pediatr. Surg. 2021, 31, 439–444.
- Sawleshwarkar, K.; Singh, M.; Bajaj, R.; Loya, S.; Chikhlondhe, R.; Bhave, S. Implementing use of sucrose analgesia (non-pharmacological management of neonatal pain) in a standalone private facility level 3 neonatal care unit using point of care quality improvement methodology. BMJ Open Qual. 2022, 11, e001830.
- Jones, L.R. Oral feeding readiness in the neonatal intensive care unit. Neonatal Netw. 2012, 31, 148–155.
- Briere, C.-E.; McGrath, J.M.; Cong, X.; Brownell, E.; Cusson, R. Direct-breastfeeding in the neonatal intensive care unit and breastfeeding duration for premature infants. Appl. Nurs. Res. 2016, 32, 47–51.
- Academy of Breastfeeding Medicine Board of Directors. Position on breastfeeding. Breastfeed. Med. 2008, 3, 267–270.
- Pediatrics, A.A.o. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841.
- Couto, G.R.; Dias, V.; de Jesus Oliveira, I. Benefits of exclusive breastfeeding: An integrative review. Nurs. Pract. Today 2020, 7, 245–254.
- Komaroff, A.; Forest, S. Implementing a clinical protocol using breastfeeding to mitigate vaccination pain in infants. J. Pediatr. Nurs. 2020, 54, 50–57.
- Callen, J.; Pinelli, J. A review of the literature examining the benefits and challenges, incidence and duration, and barriers to breastfeeding in preterm infants. Adv. Neonatal Care Off. J. Natl. Assoc. Neonatal Nurses 2005, 5, 72–88.
- Terry, J. Teaching mothers to express and store breast milk. J. Fam. Health Care 2004, 14, 121–123.
- Benoit, B.; Martin-Misener, R.; Latimer, M.; Campbell-Yeo, M. Breast-Feeding Analgesia in Infants: An Update on the Current State of Evidence. J. Perinat. Neonatal Nurs. 2017, 31, 145–159.
- Napiórkowska-Orkisz, M.; Gutysz-Wojnicka, A.; Tanajewska, M.; Sadowska-Krawczenko, I. Evaluation of Methods to Minimize Pain in Newborns during Capillary Blood Sampling for Screening: A Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 870.
- Franck, L.S.; Oulton, K.; Bruce, E. Parental involvement in neonatal pain management: An empirical and conceptual update. J. Nurs. Scholarsh. 2012, 44, 45–54.
- Skene, C.; Franck, L.; Curtis, P.; Gerrish, K. Parental involvement in neonatal comfort care. J. Obstet. Gynecol. Neonatal Nurs. 2012, 41, 786–797.
- Pölkki, T.; Korhonen, A.; Laukkala, H. Parents’ Use of Nonpharmacologic Methods to Manage Procedural Pain in Infants. J. Obstet. Gynecol. Neonatal Nurs. 2018, 47, 43–51.
- Axelin, A.; Anderzén-Carlsson, A.; Eriksson, M.; Pölkki, T.; Korhonen, A.; Franck, L.S. Neonatal Intensive Care Nurses’ Perceptions of Parental Participation in Infant Pain Management: A Comparative Focus Group Study. J. Perinat. Neonatal Nurs. 2015, 29, 363–374.
- Gates, A.; Shave, K.; Featherstone, R.; Buckreus, K.; Ali, S.; Scott, S.D.; Hartling, L. Procedural Pain: Systematic Review of Parent Experiences and Information Needs. Clin. Pediatr. 2018, 57, 672–688.
- Maciel, H.I.A.; Costa, M.F.; Costa, A.C.L.; Marcatto, J.d.O.; Manzo, B.F.; Bueno, M. Pharmacological and nonpharmacological measures of pain management and treatment among neonates. Rev. Bras. Ter. Intensiv. 2019, 31, 21–26.
- Farnia, M.R.; Babaei, R.; Shirani, F.; Momeni, M.; Hajimaghsoudi, M.; Vahidi, E.; Saeedi, M. Analgesic effect of paracetamol combined with low-dose morphine versus morphine alone on patients with biliary colic: A double blind, randomized controlled trial. World J. Emerg. Med. 2016, 7, 25–29.
- de Graaf, J.; van Lingen, R.A.; Valkenburg, A.J.; Weisglas-Kuperus, N.; Jebbink, L.G.; Wijnberg-Williams, B.; Anand, K.J.S.; Tibboel, D.; van Dijk, M. Does neonatal morphine use affect neuropsychological outcomes at 8 to 9 years of age? Pain 2013, 154, 449–458.
- Rozé, J.C.; Denizot, S.; Carbajal, R.; Ancel, P.Y.; Kaminski, M.; Arnaud, C.; Truffert, P.; Marret, S.; Matis, J.; Thiriez, G.; et al. Prolonged sedation and/or analgesia and 5-year neurodevelopment outcome in very preterm infants: Results from the EPIPAGE cohort. Arch. Pediatr. Adolesc. Med. 2008, 162, 728–733.
- Bellù, R.; Romantsik, O.; Nava, C.; de Waal, K.A.; Zanini, R.; Bruschettini, M. Opioids for newborn infants receiving mechanical ventilation. Cochrane Database Syst. Rev. 2021, 3, CD013732.
- Abushanab, D.; Abounahia, F.F.; Alsoukhni, O.; Abdelaal, M.; Al-Badriyeh, D. Clinical and Economic Evaluation of the Impact of Midazolam on Morphine Therapy for Pain Relief in Critically III Ventilated Infants with Respiratory Distress Syndrome. Pediatr. Drugs 2021, 23, 143–157.
This entry is offline, you can click here to edit this entry!
