Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Multisensory integration refers to sensory inputs from different sensory modalities being processed simultaneously to produce a unitary output. Surrounded by stimuli from multiple modalities, animals utilize multisensory integration to form a coherent and robust representation of the complex environment. Many interesting paradigms of multisensory integration have been characterized in C. elegans, for which input convergence occurs at the sensory neuron or the interneuron level.
- multisensory integration
- Caenorhabditis elegans
- sensory processing
1. General Introduction
Multisensory integration is an essential issue in the fields of cognition, behavioral science and neurobiology. It studies how information from different modalities, such as sight, sound, smell, taste and touch, becomes integrated as a coherently meaningful representation in the nervous system [1]. Successful integration can decrease sensory uncertainty and reaction latency and form better memory and perception [1], thus providing adaptive advantages for survival and reproduction.
Although sensory processing was traditionally viewed and studied in modality-specific manners, different regions of the mammalian brain are enormously interactional. Numerous studies have identified multisensory neurons in cortical areas that were previously classified as uni-sensory [2]. Multisensory integration is probably necessary for almost all animal activities. Ample evidence demonstrates that multisensory inputs are commonly found in many ascending pathways [2][3]. This leads to researchers proposing that “the entire cortex (brain?) is multisensory” [1][2][4][5][6], albeit the functional roles of the integration have not all been characterized.
There are two well-accepted principles of multisensory integration: the temporal and spatial principle and the inverse effectiveness principle [2][7][8][9]. The spatial and temporal principle states that integration is more likely to happen or be strengthened when the uni-sensory stimuli occur at approximately the same location or close in time. The principle of inverse effectiveness states that the magnitude of integration is inversely related to the responsiveness of individual stimuli, i.e., weak stimuli presented in isolation are more likely to elicit or strengthen multisensory integration [9][10][11].
The ability to integrate cross-modal senses is not inherent. Instead, it develops gradually after birth with the presence of cross-modal events in the environment. Even though multisensory neurons are produced early in life, these neurons cannot integrate multisensory inputs until much later [12]. This capability gradually matures into young adulthood. Therefore, multisensory integration is essentially a learned ability, involving the neural mechanism of plasticity.
Multisensory processing appears to be disrupted in several neuropsychiatric disorders, including autism spectrum disorder, dyslexia, attention deficit hyperactivity disorder, sensory processing disorder and schizophrenia [13][14][15][16][17][18]. How multisensory processing relates to these disorders is still unclear. It has been shown that multisensory training can restore visual function in visual cortex-damaged animals [2]. In some cases of autism, the delayed development of multisensory processing can be improved with age, presumably via prolonged development [19]. Since sensory integration intervention is based on neural plasticity [20], this gives hope that individually tailored multisensory training techniques can ameliorate these neuropsychiatric disorders with multisensory processing deficits.
Caenorhabditis elegans (C. elegans) senses its complex environment using multisensory integration strategies to make behavioral decisions [21][22]. Studies of multisensory integration in C. elegans have a unique niche due to the intrinsic properties of this organism’s nervous system. There are many advantages to studying neurobiology in C. elegans, which can be extended to the study of multisensory integration. C. elegans has a well-defined and compact nervous system with 302 neurons and it is the only organism whose entire neuronal connectome is mapped throughout different developmental stages [23][24][25]. Recently, the worm “contactome” has also been mapped, adding spatial context to the connectome [26][27]. In addition, gene expression profiles at single cell resolution of all 302 neurons have been generated [28][29].
Moreover, numerous genetic tools for neuronal functional studies have been developed in C. elegans. A single or a few neurons can be selectively killed by laser ablation [30], by expressing caspase to induce apoptosis [31], or by using miniSOG, a photosensitizer generating singlet oxygen [32][33] in a cell type-specific manner. Neuronal activity can be manipulated opto-genetically [34] or chemo-genetically [35]. Those tools greatly facilitate the identification of an underlying neural circuit. Moreover, there is an arsenal of worm mutants in various membrane potential-associated proteins, synaptic and signaling proteins, along with the ease of generating transgenic and knock-out animals, facilitating the investigation of molecular functions of the nervous system.
2. Multisensory Integration in C. elegans
2.1. Sensory Processing in C. elegans
C. elegans has 60 sensory neurons that can sense a variety of sensory modalities, including smell, taste, touch, temperature, light, color, oxygen, CO2, humidity, proprioception, magnetic field and sound [36][37][38][39][40][41][42][43][44][45]. For each environmental stimulus assayed in isolation, the fundamental neural circuit is well characterized [28] and the corresponding behavioral output is generally robust.
Worms use diverse protein receptors to sense environmental stimuli. The C. elegans genome encodes over 1000 predicted G protein-coupled receptors (GPCRs), many of which are likely to function as receptors in sensory neurons [37]. The one confirmed odorant receptor is ODR-10, which detects diacetyl [46]. GPCR LITE-1 has been shown to be a photoreceptor [47]. It has been demonstrated that the receptor guanylyl cyclase GCY-35 is an oxygen sensor [48]. Several receptor guanylyl cyclases and a glutamate receptor have been proposed as thermo-receptors [49][50]. The mechano-sensor is thought to be made up of two ion channel subunits, MEC-4 and MEC-10, from the degenerin/epithelial Na+ channel (DEG/ENaC) family [51][52].
When the GPCR protein receptors are activated by a stimulus, the signal is transduced by two types of downstream ion channels [37][38]. One type consists of the TRP (transient receptor potential) channels, OSM-9 and OCR-2 [53][54]. The other type of downstream signaling transduction is mediated by the second messenger cGMP, involving receptor guanylyl cyclases and cyclic nucleotide-gated channels TAX-4 and TAX-2 [55][56]. Both types of channels can mobilize calcium, open voltage-gated calcium channels and activate the sensory neuron.
The organization of the sensory system from all modalities is vastly different in C. elegans compared to mammals due to its numerical simplicity. Take the olfactory sensory neurons, for example. In C. elegans, a pair of each AWA, AWB and AWC neurons serve as the primary odorant chemosensory neurons, while worms are likely to express around 100 GPCRs as presumed odorant receptors [57]. Therefore, each odorant-sensing neuron expresses many receptors. This is in contrast to the “one neuron, one receptor” rule in mammals, which refers to the fact that each olfactory sensory neuron expresses one and only one olfactory receptor [58]. In the ascending pathways beyond the sensory neuron layer, the sensory systems in mammals are much more complex. Their projections travel a long distance and project to multiple higher brain regions. In C. elegans, interneurons comprise the largest group of neurons, which is probably the counterpart of the higher brain regions in mammals [24]. They can be divided into first-layer, second-layer and commander interneurons. Sensory neurons project to different layers of interneurons and converge into five commander interneurons that control muscle movement [59].
2.2. C. elegans Performs Multisensory Integration
All animals, including lower organisms such as C. elegans, can integrate information from multiple channels to form an accurate presentation of the complex environment. The integration process allows animals to make better choices based on the information they have received. The environment of C. elegans may contain both beneficial elements such as mates and food, but also harmful elements such as poison and predators. How to integrate environmental cues in a context-dependent manner and make an appropriate decision is a central theme in the studies of C. elegans neurobiology. Despite having just 60 sensory neurons, C. elegans exhibits an array of highly sensitive sensory modalities and displays diverse paradigms of multisensory integration [21][22]. These paradigms can probably be divided into two categories: (1) exposing C. elegans to two sensory modalities of opposing valence and studying how worms make decisions; (2) exposing C. elegans to stimuli from two sensory modalities and examining how the behavior evoked by one stimulus is altered by a second stimulus. All the paradigms found in C. elegans seem to be consistent in that multisensory integration can change perception.
Processing various sensory inputs at the level of sensory neurons or sensilla in the periphery is one way to accomplish multisensory integration. It can also be accomplished by integrating at the interneuron or central nervous system levels. In addition, an animal’s internal state and past experiences can top-down alter the output of sensory-evoked behavior. Below is a detailed discussion of C. elegans’ integration paradigms and top-down mechanisms.
Theoretically, two stimuli from the same sensory modality, for example, two different odorants, can also interact with each other. This scenario does not seem to be included in studies of multisensory integration in mammals but is often studied in C. elegans, providing many interesting sensory integration paradigms. In evolution, sensory integration from the same modality is likely to be fundamental to sensory integration from multiple modalities [12]. It has been found that low concentrations of different odorants often have a synergistic effect in mice [60]. This is reminiscent of the principle of inverse effectiveness. Therefore, some paradigms demonstrating sensory integration from the same modality in C. elegans will also be discussed below.
2.3. Integration at the Level of Sensory Neurons
Many organisms contain polymodal sensory neurons, meaning that those neurons can each sense multiple stimuli from different sensory modalities. In that case, polymodal sensory neurons can easily integrate sensory information from different modalities. Although sensory neurons are highly specialized in mammals, polymodal sensory neurons do exist, as exemplified by cutaneous C-fiber nociceptors [61][62]. They can respond to more than one type of noxious stimuli applied to the skin, usually mechanical, chemical and thermal [61][62]. Studying these polymodal nociceptors has provided great significance in pain management [63].
Many sensory neurons in C. elegans are polymodal. For example, the ASH neuron pair is the main nociceptor sensory neuron, which mediates avoidance responses to noxious stimuli [37]. It can sense an array of aversive cues, such as high osmolality, quinine, nose touch, repellent chemicals, heavy metals, and so on. Interestingly, after ASH activation, C. elegans can separately process stimuli from different modalities by innovating different downstream postsynaptic receptors [64]. Although high osmolality and nose touch both activate ASH neurons, high osmolality utilizes both non-NMDA and NMDA receptor subunits to mediate the avoidance response, whereas nose touch only triggers non-NMDA receptors post-synaptically [64][65]. Genetic and electrophysiological analysis suggests that this modality-specific signal transduction is because high osmolality enables increased glutamate released from ASH neurons, which is sufficient to activate both non-NMDA and NMDA receptors [65].
In addition to ASH, many other sensory neurons in C. elegans are also polymodal. For example, the chemosensory AWC neuron pair can respond to temperature [66][67]. Similarly, the AFD neuron pair primarily senses temperature but can also respond to CO2 [68][69]. These polymodal neurons all have the ability to mediate multisensory integration (Figure 1A).
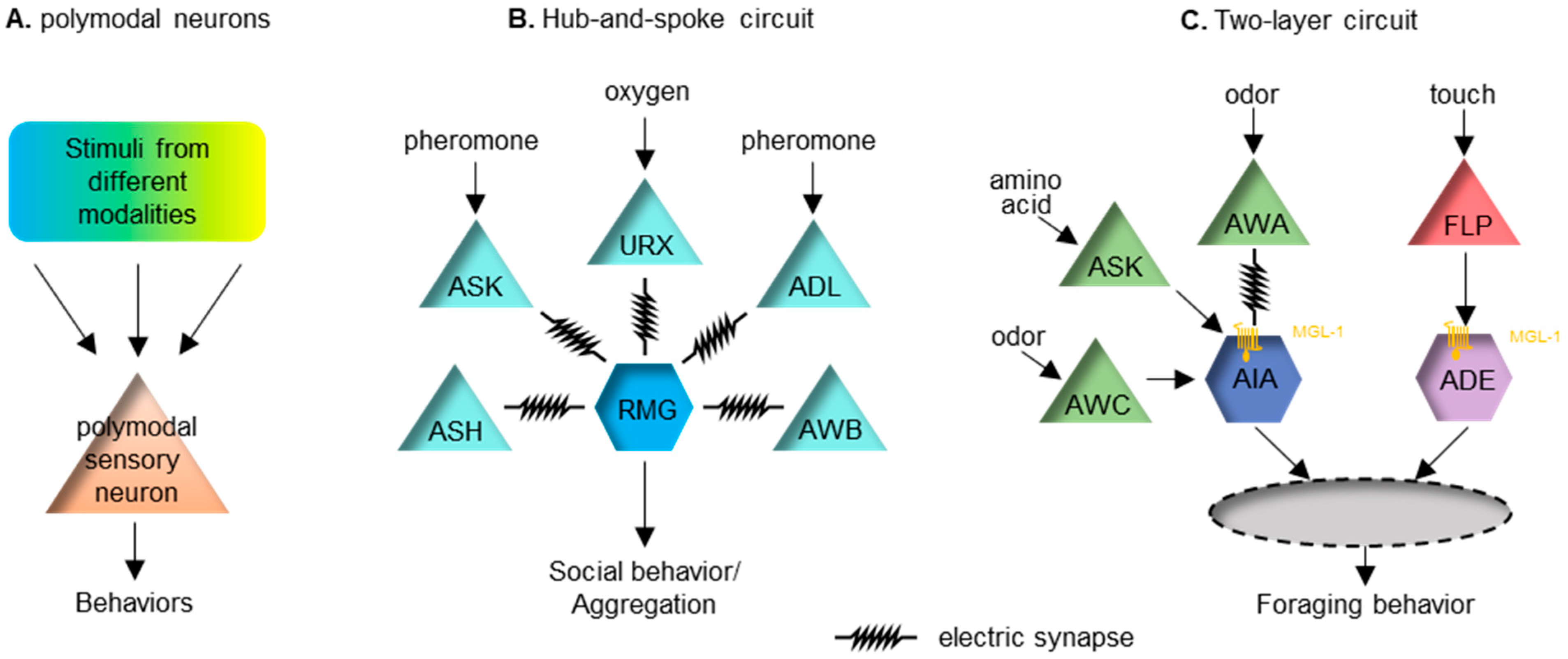
Figure 1. Several paradigms of multisensory integration in C. elegans. (A) Polymodal sensory neurons can receive and integrate inputs from different modalities. (B) A hub-and-spoke circuit. The hub neuron RMG is connected with pheromone-sensing neurons ASK and ADL, the oxygen-sensing neuron URX and several other sensory neurons via gap junctions. This circuit can integrate sensory inputs from and regulate social or aggregation behavior in C. elegans. (C) A two-layer circuit. Food-related chemosensory cues and mechanosensory cues are first integrated in parallel at the interneuron AIA and ADE, respectively, through the inhibitory metabotropic glutamate receptor MGL-1 (as symbolized by a yellow transmembrane protein), expressed post-synatpically in AIA and ADE. Additionally, glutamate can activate inhibitory ionotropic glutamate receptors in AIA. Signals from AIA and ADE will converge at the next level of the neural circuit to regulate foraging behavior in C. elegans.
In mammalian studies, multisensory integration is generally referred to as integration that occurs at the level of the sensory cortex or higher, which is beyond the first synapse in an ascending pathway [12]. Nonetheless, polymodal sensory neurons are an efficient way for stimuli from multiple modalities to be integrated through facilitation or inhibition.
2.4. Integration at the Level of Interneurons
Multisensory encoding in mammals takes place in many higher brain regions, such as the superior colliculus (SC) in the midbrain and many regions in the cerebral cortex [6][70]. Due to the significant restriction on the number of neurons, C. elegans often encodes the valance of a stimulus at the sensory neuron level [71]. Nonetheless, many paradigms of multisensory integration occur at the first- and second-layer interneurons to modulate the sensory output.
The hub-and-spoke circuit is a well-known sensory integration paradigm. One of these regulates the worm’s social behavior, or aggregation. In this circuit, the interneuron RMG acts as the hub, linking to multiple sensory neurons (the spokes) with gap junctions [72]. High activity in the RMG is essential for promoting social aggregation, of which the activity level can be modulated by several spoke neurons that sense diverse stimuli, including oxygen, sex pheromones and noxious chemicals (Figure 1B). This circuit connection motif integrates cross-modal sensory inputs to ensure a coherent output. Another similar hub-and-spoke circuit regulates nose touch response [73][74][75]. This involves the interneuron RIH being the hub connecting to sensory neurons ASH, FLP and OLQ responding to gentle touch via gap junctions.
Other interneurons can also serve as the node in a circuit. Interneuron AIA can receive inputs from many chemosensory neurons. AIA receives excitatory input from an electrical synapse and disinhibitory inputs via chemical synapses [76]. The two types of inputs need to happen coincidently to improve the reliability of AIA’s response [76]. The logic of this integrating neuron seems to relate closely to the temporal principle of multisensory integration.
Recently, a two-layer integration has been reported to modulate foraging behavior in C. elegans [77]. Forage is a stereotyped local search behavior looking for food. The behavior requires redundant inhibitory inputs from two interneuron pairs, AIA and ADE, which receive chemosensory and mechanosensory food-related cues, respectively [77]. Sensory cues symbolizing food are first organized into the chemosensory cues that are integrated at AIA and the mechanosensory cues that are integrated at ADE. Input from these two neurons subsequently integrates into the next layer of interneurons. Local search behavior can be triggered when either of these two sensory cues is removed (Figure 1C).
2.5. Top-Down Mechanisms in the Multisensory Integration
Sensory information transduction is thought to follow through a hierarchy of brain areas that are progressively more complex. “Top-down” refers to the influences of complex information from higher brain regions that shapes early sensory processing steps. Top-down influences can affect sensory processing at all cortical and thalamic levels [78]. Common top-down modulators of sensory processing can include stress, attention, expectation, emotion, motivation and learned experience [78][79][80][81].
Although C. elegans lacks cognition and emotion, the sensory output can be influenced by its past experience and internal physiological states, such as hunger and sickness. The most well-studied top-down modulator in C. elegans is probably starvation, likely to be due to a lack of other top-down cognitive or emotional modulators. Hunger will increase C. elegans’ preference for seeking attractive odors cueing for food availability in the risk of other harmful stimuli [82][83][84].
In a risk-reward choice assay [82], C. elegans is trapped inside a circle of a repulsive hyperosmotic fructose solution, while an attractive food odor is placed outside the circle. The outcome is scored on whether worms cross the aversive circle to reach the attractive odor. Almost no worms would exit the circle in the initial 15 min. However, after being starved for 5 h, almost 80% of the worms would exit the repulsive circle, seeking the attractive odor. The interneuron RIM is identified as modulating this decision via a top-down extra-synaptic aminergic signal [82]. In another scenario of multisensory integration between opposing valences, the insulin/IGF-1 signaling (IIS) pathway is mediating the signal of hunger to decrease responses to the repellent gustatory cue [84]. Several other neuromodulators have also been found to relay the signal of starvation to functionally reconfigure sensory processing and, presumably, they can also mediate top-down regulation impinging upon multisensory integration.
Past experience is another well-studied top-down modulator for sensory processing in C. elegans. A recent study demonstrated how worms can learn to navigate a T-maze to locate food via multisensory cues [85]. In general, past experience affects sensory processing via reshaping the synapse. Here, researchers provide two examples to demonstrate how prior experience can change either the strength or the composition of the synapse to enable plasticity. C. elegans does not have an innately preferred temperature. Instead, it remembers its cultivation temperature and moves to that temperature when subjected to a temperature gradient [86]. This sensory memory is encoded by the synaptic strength between the thermo-sensory neuron pair AFD and its downstream interneuron AIY [87]. Under warmer temperatures, this synapse is strengthened, enabling worms to move to warmth and vice versa. Similarly, C. elegans cultivated at a certain NaCl concentration can remember this concentration and travel to it when subjected to a NaCl gradient [88]. This gustatory memory is encoded by differentially innervating the glutamate receptors in the AIB neuron, which is postsynaptic to the salt-sensing neuron ASE right (ASER). At a higher salt cultivation condition, decreasing NaCl concentration causes ASER activation, triggers glutamate released from ASER and subsequently activates the excitatory glutamate receptor GLR-1 in the downstream AIB neurons, whereas, cultivated in a lower salt environment, glutamate released from ASER activates the inhibitory glutamate receptor AVR-14 in AIB instead [89].
This entry is adapted from the peer-reviewed paper 10.3390/brainsci12101368
References
- Stein, B.E.; Stanford, T.R. Multisensory Integration: Current Issues from the Perspective of the Single Neuron. Nat. Rev. Neurosci. 2008, 9, 255–266.
- Stein, B.E.; Stanford, T.R.; Rowland, B.A. Multisensory Integration and the Society for Neuroscience: Then and Now. J. Neurosci. 2020, 40, 3–11.
- Paraskevopoulos, E.; Herholz, S. Multisensory Integration and Neuroplasticity in the Human Cerebral Cortex. Transl. Neurosci. 2013, 4, 337–348.
- Driver, J.; Noesselt, T. Multisensory Interplay Reveals Crossmodal Influences on “sensory-Specific” Brain Regions, Neural Responses, and Judgments. Neuron 2008, 57, 11–23.
- Ghazanfar, A.A.; Schroeder, C.E. Is Neocortex Essentially Multisensory? Trends Cogn. Sci. 2006, 10, 278–285.
- Yau, J.M.; DeAngelis, G.C.; Angelaki, D.E. Dissecting Neural Circuits for Multisensory Integration and Crossmodal Processing. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140203.
- Buchholz, V.N.; Goonetilleke, S.C.; Medendorp, W.P.; Corneil, B.D. Greater Benefits of Multisensory Integration during Complex Sensorimotor Transformations. J. Neurophysiol. 2012, 107, 3135–3143.
- Meredith, M.A.; Stein, B.E. Spatial Factors Determine the Activity of Multisensory Neurons in Cat Superior Colliculus. Brain Res. 1986, 365, 350–354.
- Meredith, M.A.; Stein, B.E. Visual, Auditory, and Somatosensory Convergence on Cells in Superior Colliculus Results in Multisensory Integration. J. Neurophysiol. 1986, 56, 640–662.
- Meredith, M.A.; Stein, B.E. Interactions among Converging Sensory Inputs in the Superior Colliculus. Science 1983, 221, 389–391.
- Holmes, N.P. The Principle of Inverse Effectiveness in Multisensory Integration: Some Statistical Considerations. Brain Topogr. 2009, 21, 168–176.
- Stein, B.E.; Stanford, T.R.; Rowland, B.A. Development of Multisensory Integration from the Perspective of the Individual Neuron. Nat. Rev. Neurosci. 2014, 15, 520–535.
- Hornix, B.E.; Havekes, R.; Kas, M.J.H. Multisensory Cortical Processing and Dysfunction across the Neuropsychiatric Spectrum. Neurosci. Biobehav. Rev. 2019, 97, 138–151.
- Marco, E.J.; Hinkley, L.B.N.; Hill, S.S.; Nagarajan, S.S. Sensory Processing in Autism: A Review of Neurophysiologic Findings. Pediatr. Res. 2011, 69, 48R–54R.
- Stevenson, R.A.; Park, S.; Cochran, C.; McIntosh, L.G.; Noel, J.-P.; Barense, M.D.; Ferber, S.; Wallace, M.T. The Associations between Multisensory Temporal Processing and Symptoms of Schizophrenia. Schizophr. Res. 2017, 179, 97–103.
- Stevenson, R.A.; Segers, M.; Ferber, S.; Barense, M.D.; Wallace, M.T. The Impact of Multisensory Integration Deficits on Speech Perception in Children with Autism Spectrum Disorders. Front Psychol. 2014, 5, 379.
- Panagiotidi, M.; Overton, P.G.; Stafford, T. Multisensory Integration and ADHD-like Traits: Evidence for an Abnormal Temporal Integration Window in ADHD. Acta Psychol. 2017, 181, 10–17.
- Zvyagintsev, M.; Parisi, C.; Mathiak, K. Temporal Processing Deficit Leads to Impaired Multisensory Binding in Schizophrenia. Cogn. Neuropsychiatry 2017, 22, 361–372.
- Beker, S.; Foxe, J.J.; Molholm, S. Ripe for Solution: Delayed Development of Multisensory Processing in Autism and Its Remediation. Neurosci. Biobehav. Rev. 2018, 84, 182–192.
- Cheung, P.P.P.; Lau, B.W.M. Chapter Six—Neurobiology of Sensory Processing in Autism Spectrum Disorder. In Progress in Molecular Biology and Translational Science; Ilieva, M., Lau, W.K.-W., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 173, pp. 161–181.
- Ghosh, D.D.; Nitabach, M.N.; Zhang, Y.; Harris, G. Multisensory Integration in C. elegans. Curr. Opin. Neurobiol. 2017, 43, 110–118.
- Metaxakis, A.; Petratou, D.; Tavernarakis, N. Multimodal Sensory Processing in Caenorhabditis elegans. Open Biol. 2018, 8, 180049.
- White, J.G.; Southgate, E.; Thomson, J.N.; Brenner, S. The Structure of the Nervous System of the Nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1986, 314, 1–340.
- Cook, S.J.; Jarrell, T.A.; Brittin, C.A.; Wang, Y.; Bloniarz, A.E.; Yakovlev, M.A.; Nguyen, K.C.Q.; Tang, L.T.-H.; Bayer, E.A.; Duerr, J.S.; et al. Whole-Animal Connectomes of Both Caenorhabditis elegans Sexes. Nature 2019, 571, 63–71.
- Witvliet, D.; Mulcahy, B.; Mitchell, J.K.; Meirovitch, Y.; Berger, D.R.; Wu, Y.; Liu, Y.; Koh, W.X.; Parvathala, R.; Holmyard, D.; et al. Connectomes across Development Reveal Principles of Brain Maturation. Nature 2021, 596, 257–261.
- Moyle, M.W.; Barnes, K.M.; Kuchroo, M.; Gonopolskiy, A.; Duncan, L.H.; Sengupta, T.; Shao, L.; Guo, M.; Santella, A.; Christensen, R.; et al. Structural and Developmental Principles of Neuropil Assembly in C. elegans. Nature 2021, 591, 99–104.
- Brittin, C.A.; Cook, S.J.; Hall, D.H.; Emmons, S.W.; Cohen, N. A Multi-Scale Brain Map Derived from Whole-Brain Volumetric Reconstructions. Nature 2021, 591, 105–110.
- Hammarlund, M.; Hobert, O.; Miller, D.M.; Sestan, N. The CeNGEN Project: The Complete Gene Expression Map of an Entire Nervous System. Neuron 2018, 99, 430–433.
- Taylor, S.R.; Santpere, G.; Weinreb, A.; Barrett, A.; Reilly, M.B.; Xu, C.; Varol, E.; Oikonomou, P.; Glenwinkel, L.; McWhirter, R.; et al. Molecular Topography of an Entire Nervous System. Cell 2021, 184, 4329–4347.e23.
- Bargmann, C.I.; Avery, L. Laser Killing of Cells in Caenorhabditis elegans. Methods Cell Biol. 1995, 48, 225–250.
- Chelur, D.S.; Chalfie, M. Targeted Cell Killing by Reconstituted Caspases. Proc. Natl. Acad. Sci. USA 2007, 104, 2283–2288.
- Qi, Y.B.; Garren, E.J.; Shu, X.; Tsien, R.Y.; Jin, Y. Photo-Inducible Cell Ablation in Caenorhabditis elegans Using the Genetically Encoded Singlet Oxygen Generating Protein MiniSOG. Proc. Natl. Acad. Sci. USA 2012, 109, 7499–7504.
- Xu, S.; Chisholm, A.D. Highly Efficient Optogenetic Cell Ablation in C. elegans Using Membrane-Targeted MiniSOG. Sci. Rep. 2016, 6, 21271.
- Bergs, A.; Schultheis, C.; Fischer, E.; Tsunoda, S.P.; Erbguth, K.; Husson, S.J.; Govorunova, E.; Spudich, J.L.; Nagel, G.; Gottschalk, A.; et al. Rhodopsin Optogenetic Toolbox v2.0 for Light-Sensitive Excitation and Inhibition in Caenorhabditis elegans. PLoS ONE 2018, 13, e0191802.
- Pokala, N.; Liu, Q.; Gordus, A.; Bargmann, C.I. Inducible and Titratable Silencing of Caenorhabditis elegans Neurons in Vivo with Histamine-Gated Chloride Channels. Proc. Natl. Acad. Sci. USA 2014, 111, 2770–2775.
- Russell, J.; Vidal-Gadea, A.G.; Makay, A.; Lanam, C.; Pierce-Shimomura, J.T. Humidity Sensation Requires Both Mechanosensory and Thermosensory Pathways in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2014, 111, 8269–8274.
- Bargmann, C.I. Chemosensation in C. elegans. WormBook 2006, 1–29.
- Ferkey, D.M.; Sengupta, P.; L’Etoile, N.D. Chemosensory Signal Transduction in Caenorhabditis elegans. Genetics 2021, 217, iyab004.
- Goodman, M.B. Mechanosensation. WormBook 2006, 1–14.
- Goodman, M.B.; Klein, M.; Lasse, S.; Luo, L.; Mori, I.; Samuel, A.; Sengupta, P.; Wang, D. Thermotaxis Navigation Behavior. WormBook 2014, 1–10.
- Ward, A.; Liu, J.; Feng, Z.; Xu, X.Z.S. Light-Sensitive Neurons and Channels Mediate Phototaxis in C. elegans. Nat. Neurosci. 2008, 11, 916–922.
- Ghosh, D.D.; Lee, D.; Jin, X.; Horvitz, H.R.; Nitabach, M.N. C. elegans Discriminates Colors to Guide Foraging. Science 2021, 371, 1059–1063.
- Wen, Q.; Po, M.D.; Hulme, E.; Chen, S.; Liu, X.; Kwok, S.W.; Gershow, M.; Leifer, A.M.; Butler, V.; Fang-Yen, C.; et al. Proprioceptive Coupling within Motor Neurons Drives C. elegans Forward Locomotion. Neuron 2012, 76, 750–761.
- Vidal-Gadea, A.; Ward, K.; Beron, C.; Ghorashian, N.; Gokce, S.; Russell, J.; Truong, N.; Parikh, A.; Gadea, O.; Ben-Yakar, A.; et al. Magnetosensitive Neurons Mediate Geomagnetic Orientation in Caenorhabditis elegans. eLife 2015, 4, e07493.
- Iliff, A.J.; Wang, C.; Ronan, E.A.; Hake, A.E.; Guo, Y.; Li, X.; Zhang, X.; Zheng, M.; Liu, J.; Grosh, K.; et al. The Nematode C. elegans Senses Airborne Sound. Neuron 2021, 109, 3633–3646.e7.
- Sengupta, P.; Chou, J.H.; Bargmann, C.I. Odr-10 Encodes a Seven Transmembrane Domain Olfactory Receptor Required for Responses to the Odorant Diacetyl. Cell 1996, 84, 899–909.
- Gong, J.; Yuan, Y.; Ward, A.; Kang, L.; Zhang, B.; Wu, Z.; Peng, J.; Feng, Z.; Liu, J.; Xu, X.Z.S. The C. elegans Taste Receptor Homolog LITE-1 Is a Photoreceptor. Cell 2016, 167, 1252–1263.e10.
- Gray, J.M.; Karow, D.S.; Lu, H.; Chang, A.J.; Chang, J.S.; Ellis, R.E.; Marletta, M.A.; Bargmann, C.I. Oxygen Sensation and Social Feeding Mediated by a C. elegans Guanylate Cyclase Homologue. Nature 2004, 430, 317–322.
- Gong, J.; Liu, J.; Ronan, E.A.; He, F.; Cai, W.; Fatima, M.; Zhang, W.; Lee, H.; Li, Z.; Kim, G.-H.; et al. A Cold-Sensing Receptor Encoded by a Glutamate Receptor Gene. Cell 2019, 178, 1375–1386.e11.
- Takeishi, A.; Yu, Y.V.; Hapiak, V.M.; Bell, H.W.; O’Leary, T.; Sengupta, P. Receptor-Type Guanylyl Cyclases Confer Thermosensory Responses in C. elegans. Neuron 2016, 90, 235–244.
- Goodman, M.B.; Ernstrom, G.G.; Chelur, D.S.; O’Hagan, R.; Yao, C.A.; Chalfie, M. MEC-2 Regulates C. elegans DEG/ENaC Channels Needed for Mechanosensation. Nature 2002, 415, 1039–1042.
- O’Hagan, R.; Chalfie, M.; Goodman, M.B. The MEC-4 DEG/ENaC Channel of Caenorhabditis elegans Touch Receptor Neurons Transduces Mechanical Signals. Nat. Neurosci. 2005, 8, 43–50.
- Colbert, H.A.; Smith, T.L.; Bargmann, C.I. OSM-9, A Novel Protein with Structural Similarity to Channels, Is Required for Olfaction, Mechanosensation, and Olfactory Adaptation in Caenorhabditis elegans. J. Neurosci. 1997, 17, 8259–8269.
- Tobin, D.M.; Madsen, D.M.; Kahn-Kirby, A.; Peckol, E.L.; Moulder, G.; Barstead, R.; Maricq, A.V.; Bargmann, C.I. Combinatorial Expression of TRPV Channel Proteins Defines Their Sensory Functions and Subcellular Localization in C. elegans Neurons. Neuron 2002, 35, 307–318.
- Coburn, C.M.; Bargmann, C.I. A Putative Cyclic Nucleotide–Gated Channel Is Required for Sensory Development and Function in C. elegans. Neuron 1996, 17, 695–706.
- Komatsu, H.; Mori, I.; Rhee, J.-S.; Akaike, N.; Ohshima, Y. Mutations in a Cyclic Nucleotide–Gated Channel Lead to Abnormal Thermosensation and Chemosensation in C. elegans. Neuron 1996, 17, 707–718.
- Troemel, E.R.; Chou, J.H.; Dwyer, N.D.; Colbert, H.A.; Bargmann, C.I. Divergent Seven Transmembrane Receptors Are Candidate Chemosensory Receptors in C. elegans. Cell 1995, 83, 207–218.
- Serizawa, S.; Miyamichi, K.; Sakano, H. One Neuron-One Receptor Rule in the Mouse Olfactory System. Trends Genet. 2004, 20, 648–653.
- Chalfie, M.; Sulston, J.E.; White, J.G.; Southgate, E.; Thomson, J.N.; Brenner, S. The Neural Circuit for Touch Sensitivity in Caenorhabditis elegans. J. Neurosci. 1985, 5, 956–964.
- Inagaki, S.; Iwata, R.; Iwamoto, M.; Imai, T. Widespread Inhibition, Antagonism, and Synergy in Mouse Olfactory Sensory Neurons In Vivo. Cell Rep. 2020, 31, 107814.
- Kumazawa, T. Chapter 1. The Polymodal Receptor: Bio-Warning and Defense System. In Progress in Brain Research; Kumazawa, T., Kruger, L., Mizumura, K., Eds.; The Polymodal Pathological Pain Receptor—A Gateway to Pathological Pain; Elsevier: Amsterdam, The Netherlands, 1996; Volume 113, pp. 3–18.
- Lawson, S.N.; Fang, X.; Djouhri, L. Nociceptor Subtypes and Their Incidence in Rat Lumbar Dorsal Root Ganglia (DRGs): Focussing on C-Polymodal Nociceptors, Aβ-Nociceptors, Moderate Pressure Receptors and Their Receptive Field Depths. Curr. Opin. Physiol. 2019, 11, 125–146.
- Ochoa, J. Chapter 11. Human Polymodal Receptors in Pathological Conditions. In Progress in Brain Research; Kumazawa, T., Kruger, L., Mizumura, K., Eds.; The Polymodal Pathological Pain Receptor—A Gateway to Pathological Pain; Elsevier: Amsterdam, The Netherlands, 1996; Volume 113, pp. 185–197.
- Hart, A.C.; Sims, S.; Kaplan, J.M. Synaptic Code for Sensory Modalities Revealed by C. elegans GLR-1 Glutamate Receptor. Nature 1995, 378, 82–85.
- Mellem, J.E.; Brockie, P.J.; Zheng, Y.; Madsen, D.M.; Maricq, A.V. Decoding of Polymodal Sensory Stimuli by Postsynaptic Glutamate Receptors in C. elegans. Neuron 2002, 36, 933–944.
- Biron, D.; Wasserman, S.; Thomas, J.H.; Samuel, A.D.T.; Sengupta, P. An Olfactory Neuron Responds Stochastically to Temperature and Modulates Caenorhabditis elegans Thermotactic Behavior. Proc. Natl. Acad. Sci. USA 2008, 105, 11002–11007.
- Kuhara, A.; Okumura, M.; Kimata, T.; Tanizawa, Y.; Takano, R.; Kimura, K.D.; Inada, H.; Matsumoto, K.; Mori, I. Temperature Sensing by an Olfactory Neuron in a Circuit Controlling Behavior of C. elegans. Science 2008, 320, 803–807.
- Bretscher, A.J.; Kodama-Namba, E.; Busch, K.E.; Murphy, R.J.; Soltesz, Z.; Laurent, P.; de Bono, M. Temperature, Oxygen, and Salt-Sensing Neurons in C. elegans Are Carbon Dioxide Sensors That Control Avoidance Behavior. Neuron 2011, 69, 1099–1113.
- Kodama-Namba, E.; Fenk, L.A.; Bretscher, A.J.; Gross, E.; Busch, K.E.; de Bono, M. Cross-Modulation of Homeostatic Responses to Temperature, Oxygen and Carbon Dioxide in C. elegans. PLoS Genet. 2013, 9, e1004011.
- Lemus, L.; Hernández, A.; Luna, R.; Zainos, A.; Romo, R. Do Sensory Cortices Process More than One Sensory Modality during Perceptual Judgments? Neuron 2010, 67, 335–348.
- Troemel, E.R.; Kimmel, B.E.; Bargmann, C.I. Reprogramming Chemotaxis Responses: Sensory Neurons Define Olfactory Preferences in C. elegans. Cell 1997, 91, 161–169.
- Macosko, E.Z.; Pokala, N.; Feinberg, E.H.; Chalasani, S.H.; Butcher, R.A.; Clardy, J.; Bargmann, C.I. A Hub-and-Spoke Circuit Drives Pheromone Attraction and Social Behaviour in C. elegans. Nature 2009, 458, 1171–1175.
- Kaplan, J.M.; Horvitz, H.R. A Dual Mechanosensory and Chemosensory Neuron in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 1993, 90, 2227–2231.
- Rabinowitch, I.; Chatzigeorgiou, M.; Schafer, W.R. A Gap Junction Circuit Enhances Processing of Coincident Mechanosensory Inputs. Curr. Biol. 2013, 23, 963–967.
- Chatzigeorgiou, M.; Schafer, W.R. Lateral Facilitation between Primary Mechanosensory Neurons Controls Nose Touch Perception in C. elegans. Neuron 2011, 70, 299–309.
- Dobosiewicz, M.; Liu, Q.; Bargmann, C.I. Reliability of an Interneuron Response Depends on an Integrated Sensory State. eLife 2019, 8, e50566.
- López-Cruz, A.; Sordillo, A.; Pokala, N.; Liu, Q.; McGrath, P.T.; Bargmann, C.I. Parallel Multimodal Circuits Control an Innate Foraging Behavior. Neuron 2019, 102, 407–419.e8.
- Gilbert, C.D.; Sigman, M. Brain States: Top-Down Influences in Sensory Processing. Neuron 2007, 54, 677–696.
- Gilbert, C.D.; Li, W. Top-down Influences on Visual Processing. Nat. Rev. Neurosci. 2013, 14, 350–363.
- Battaglia, S.; Orsolini, S.; Borgomaneri, S.; Barbieri, R.; Diciotti, S.; di Pellegrino, G. Characterizing Cardiac Autonomic Dynamics of Fear Learning in Humans. Psychophysiology 2022, e14122.
- Gilbert, C.D.; Sigman, M.; Crist, R.E. The Neural Basis of Perceptual Learning. Neuron 2001, 31, 681–697.
- Ghosh, D.D.; Sanders, T.; Hong, S.; McCurdy, L.Y.; Chase, D.L.; Cohen, N.; Koelle, M.R.; Nitabach, M.N. Neural Architecture of Hunger-Dependent Multisensory Decision Making in C. elegans. Neuron 2016, 92, 1049–1062.
- Ishihara, T.; Iino, Y.; Mohri, A.; Mori, I.; Gengyo-Ando, K.; Mitani, S.; Katsura, I. HEN-1, a Secretory Protein with an LDL Receptor Motif, Regulates Sensory Integration and Learning in Caenorhabditis elegans. Cell 2002, 109, 639–649.
- Matty, M.A.; Lau, H.E.; Haley, J.A.; Singh, A.; Chakraborty, A.; Kono, K.; Reddy, K.C.; Hansen, M.; Chalasani, S.H. Intestine-to-Neuronal Signaling Alters Risk-Taking Behaviors in Food-Deprived Caenorhabditis elegans. PLoS Genet. 2022, 18, e1010178.
- Gourgou, E.; Adiga, K.; Goettemoeller, A.; Chen, C.; Hsu, A.-L. Caenorhabditis elegans Learning in a Structured Maze Is a Multisensory Behavior. iScience 2021, 24, 102284.
- Hedgecock, E.M.; Russell, R.L. Normal and Mutant Thermotaxis in the Nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 1975, 72, 4061–4065.
- Hawk, J.D.; Calvo, A.C.; Liu, P.; Almoril-Porras, A.; Aljobeh, A.; Torruella-Suárez, M.L.; Ren, I.; Cook, N.; Greenwood, J.; Luo, L.; et al. Integration of Plasticity Mechanisms within a Single Sensory Neuron of C. elegans Actuates a Memory. Neuron 2018, 97, 356–367.e4.
- Kunitomo, H.; Sato, H.; Iwata, R.; Satoh, Y.; Ohno, H.; Yamada, K.; Iino, Y. Concentration Memory-Dependent Synaptic Plasticity of a Taste Circuit Regulates Salt Concentration Chemotaxis in Caenorhabditis elegans. Nat. Commun. 2013, 4, 2210.
- Sato, H.; Kunitomo, H.; Fei, X.; Hashimoto, K.; Iino, Y. Glutamate Signaling from a Single Sensory Neuron Mediates Experience-Dependent Bidirectional Behavior in Caenorhabditis elegans. Cell Rep. 2021, 35, 109177.
This entry is offline, you can click here to edit this entry!
