1. Overview of Sources and Production of CNMs
Cellulose is a white, fibre-like structure consisting of a linear polysaccharide chain composed of repeated units of two D-glucopyranose rings linked together by oxygen covalently bonded to C1 of one glucose ring and C4 of the adjoining ring (β-1,4 glycosidic bond), with free hydroxyl groups (-OH) at the C-2, C-3, and C-6 atoms [
12,
28]. Cellulose materials can be produced at the nanoscale, and the resulting CNMs may be grouped into five main categories based on the cellulose source, extraction/production method, and surface chemistry [
14,
33]. These categories are cellulose nanocrystals (CNCs), cellulose nanofibrils (CNFs), cellulose nanocrystals from tunicates (t-CNCs), algal cellulose (AC), and bacterial nanocellulose (BC) [
14,
23,
33,
34].
The most common sources of CNMs—particularly of CNFs and CNCs—are cellulose obtained from plants, namely, hardwood or softwood pulp. Plant cellulose can also be extracted from seed fibres, bast fibres, grasses, banana peel, oil palm, and rice straw, among others [
13,
16,
23,
35]. Cellulose is predominantly located in the plant’s secondary cell wall, which is reinforced by a matrix also consisting of lignin, hemicellulose, pectin, proteins, extractive organic substances, and trace elements [
23,
34,
36]. Large cellulose fibres are constituted by parallel stacking of multiple cellulose chains that form the elementary fibrils and are assembled into larger microfibril bundles [
23,
34]. The elementary cellulose fibrils are constituted by regions of highly ordered cellulose chains (i.e., crystalline) that form the core, which alternate with disordered regions (i.e., amorphous) [
23,
34]. CNFs—also known as nanofibrillated cellulose, nanofibrillar cellulose, or cellulose nanofibres—are defined as “cellulose nanofibres composed of at least one elementary fibril, containing crystalline, paracrystalline and amorphous regions, with aspect ratios usually greater than 10 nm, which may contain longitudinal splits, entanglement between particles, or network-like structures” [
1]. CNFs’ dimensions are typically 3–100 nm in cross-section (diameter) and up to 100 μm in length [
1]. CNCs—also known as nanocrystalline cellulose, cellulose nanowhiskers, needles, spheres, or nanowires—are defined as “nanocrystals predominantly composed of cellulose with at least one elementary fibril containing predominantly crystalline and paracrystalline regions, with aspect ratio ranging from 5 to 50, not exhibiting longitudinal splits, inter-particle entanglement, or network-like structures” [
1]. CNCs’ dimensions are typically 3–50 nm in cross-section and 100 nm to several μm in length, depending on the source material [
1]. CNFs or CNCs are obtained by fragmentation of the cellulose hierarchical structure using different chemical, enzymatic, and/or mechanical approaches. An overview of the extraction processes for CNFs and CNCs is detailed elsewhere [
36,
37,
38]. Briefly, the production of CNFs requires breaking the fibres into delaminated individual nanofibrils. These are mainly obtained via high-energy mechanical shearing methods, such as ultrafine grinding/microgrinding, microfluidisation, high-intensity ultrasonication, or high-pressure homogenisation, among others [
3,
36,
38,
39]. These processes are normally preceded by chemical or enzymatic hydrolysis treatments to increase the fragmentation/depolymerisation (nanofibrillation) efficiency and reduce production costs [
3,
29,
36,
37,
39]. These treatments also contribute to removing non-cellulosic constituents such as lignin and hemicelluloses, producing highly purified cellulose, and can yield CNFs with modified surface chemistry [
36]. Common chemical treatments leading to chemical modifications of CNFs include catalytic oxidation with 2,2,6,6-tetramethylpiperidine-1-oxyl radicals (TEMPO oxidation), whereby the primary hydroxyl groups on the C6 position of cellulose are converted to carboxylic groups [
40,
41,
42]; carboxymethylation [
43,
44,
45]; phosphorylation [
44,
45]; etc. CNCs are derived from the crystalline regions of cellulose and are commonly extracted by acid hydrolysis of cellulose pulp using mineral acids—typically sulfuric, hydrochloric, or phosphoric acid [
33]. Acid hydrolysis removes non-cellulosic components and annihilates most of the amorphous regions, leaving the crystalline regions, and resulting in the formation of nanocrystal structures [
33,
34,
38,
46]. CNMs extracted from different sources via different production methods are analogous in chemical composition but have different morphologies, lengths, widths, aspect ratios, degrees of polymerisation (i.e., the number of glucose units), and crystallinity [
3,
14,
20,
34]. Different ratios of amorphous fractions and crystalline domains influence the CNMs’ physical characteristics. While CNCs have a short needle- or rod-like morphology demonstrating similar diameters and a high level of rigidity/stiffness due to their high crystallinity, entangled CNFs have fibre/fibril morphologies with a higher aspect ratio, plasticity, and flexibility [
23,
33].
The accessibility of hydroxyl groups on the cellulose surface and the relatively large specific surface area of CNMs offer many possibilities for their modification and functionalisation during production, resulting in different surface chemistries [
4,
14,
33,
39]. Chemical modifications can occur as a byproduct of the extraction process (e.g., sulphate half-ester formation after treatment with sulfuric acid, carboxylic acid after treatment with TEMPO, etc.) [
48]. Surface functionalisation can also occur via adsorption to the surface of the particles and covalent attachment of molecules or derivatisation of the surface [
48]. Molecular grafting, grafting of polymers or supramolecular units, the addition of fluorescent tags, and nanoparticles, among others, are often used to functionalise CNMs [
14,
45,
48,
49,
50,
51,
52]. The variety of chemistries currently being used has been summarised in recent reviews [
39,
45,
48,
53,
54]. The surface chemistry affects the CNMs’ hydrophilic/hydrophobic balance and its interaction with the surrounding environment, influencing CNMs’ degree of aggregation and agglomeration, dispersibility in solvents or polymers, rheology, and applicability in multiple systems [
14,
33,
39,
55,
56]. Moreover, surface chemistry can also influence CNMs’ interactions with biological systems [
57,
58,
59].
2. CNMs in Food Technology and Biomedicine
Conventional cellulose and some of its derivatives have a long history of use as additives in food and animal feed. In the European Union, several micron-sized or larger celluloses and cellulose derivatives are currently authorised as food additives in almost all food categories, at quantum satis (QS), as defined in Annex II of Regulation (EC) No 1333/2008 on food additives. They are considered safe for use as food additives, and an acceptable daily intake (ADI) has been considered unnecessary, based on their low toxicity, absence of genotoxic concerns and, if any, their negligible absorption in the human GIT [
60]. Several celluloses and their derivatives are also authorised under the European Regulation (EC) No 10/2011 for plastics for food packaging, as well as for use as polymer additives, production aids, and other starting substances. Conversely, CNMs have not yet been authorised as food additives or as food contact materials in Europe. Specific assessments are required for their safety evaluation in the framework of food and feed in the European Union, as described by the European Food Safety Authority (EFSA)’s guidance on nanomaterials [
61]. In the USA, celluloses have been designated “generally regarded as safe” (GRAS) by the US Food and Drug Administration (FDA) and are approved as food additives by the US Department of Agriculture’s Food Safety and Inspection Service [
62,
63]. No specific regulatory provisions are in place for pharmaceutical drugs or other medical products and devices using NMs in the USA, Canada, the UK, Japan, or Europe [
64]. In Europe, some guidelines are available for specific NMs for applications in human medicines [
65,
66].
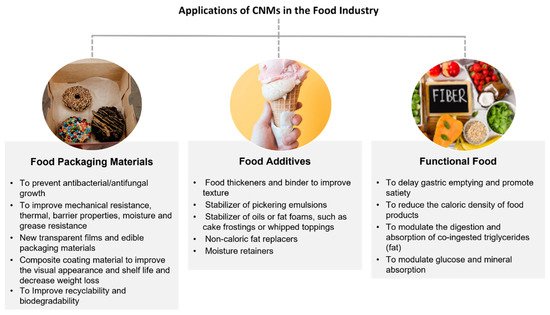
CNMs have found a multitude of potential applications in the food industry [
2,
46,
67,
68]. Three main categories of applications can be foreseen: food packaging materials, food additives, and functional foods [
46,
67], as depicted in
Figure 1.
Figure 1. Potential applications of CNMs in the food industry, including food contact materials.
There is currently a great demand for new and innovative food packaging materials driven by the policy of using fewer plastics and increasing recyclability. Replacing conventional non-renewable and practically non-biodegradable oil-based materials with CNM-based packaging materials could increase the biodegradability of food packaging and reduce packaging waste, minimising the environmental impact [
12,
68,
69]. Several nanocomposite food packaging materials containing CNMs have been developed, showing promising results in improving the food packaging’s main functions, i.e., extending food’s stability and assuring its quality and safety for a longer shelf-life [
2,
3,
12,
16,
68,
69,
70,
71,
72]. CNMs can be incorporated as reinforcing structures in different types of food packaging materials (e.g., as a filler or coating), to improve transparency or mechanical (e.g., tensile strength), thermal, and barrier (e.g., gases and water vapour) properties, as compared to conventional materials [
12,
67,
68,
71,
73,
74,
75]. When applied as coating materials in the inner layer of food packaging materials (such as paper), CNMs can contribute to protecting packaging from water (e.g., cups for coffee) or increasing its resistance to grease/oil (e.g., in pizza/hamburger boxes) [
71]. CNMs can also be added to packaging composites to increase anti-microbiological performance in various types of foods and materials [
67,
71,
74,
76,
77,
78]. Additionally, the quality of fruits and vegetables (e.g., strawberries, blueberries, pomegranate seeds, or pears) has been improved when coated with CNM composites (e.g., chitosan/CNMs or CNFs/nanoparticles of calcium carbonate), showing decreased weight loss and improved food preservation, or improved visual appearance [
79,
80,
81,
82]. Edible packaging materials made of CNM composites with either increased antimicrobial properties or improved mechanical strength or barrier properties have also been investigated [
83,
84,
85].
As food additives, CNMs have been proposed as stabilisers of oil-in-water Pickering emulsions with or without antibacterial properties, to improve food’s homogeneity and stability [
11,
86,
87,
88]. CNMs or CNM composites may be used to stabilise oils or fat foams, such as cake frostings or whipped toppings, or emulsions such as salad dressings, sauces, and gravies [
46]. They can also potentially be applied as rheology modifiers—for example, to improve gelling (i.e., anti-melting) and texture properties, as a fat substitute to reduce the caloric value of ice cream [
89,
90], or to thicken powder-based soy milk [
46]. Additionally, they have been proposed as a non-caloric fat substitute in meat sausages using nanocellulose-stabilised soybean oil to retain moisture without compromising texture [
91].
One of the main advantages of CNMs is their appeal to dietetic foods, due to their indigestibility by humans. The use of CNMs as functional foods has also been proposed, with their effects being studied in various aspects of the digestion process as a non-caloric fibre source (i.e., dietary fibre) to reduce the caloric density of food products [
9]. They can also be used as modulating agents in the digestion and absorption of co-ingested triglycerides (i.e., fat) [
10], to delay the digestion and diffusion of starch [
92], or to modulate the absorption of glucose and minerals [
92,
93].
In addition to their uses in the food industry, CNMs have been characterised as very promising materials for biomedical and pharmaceutical applications. This is due to their supposed biocompatibility, chemical modification capabilities (vide supra), water-retaining capacity, hydrophilic properties, advantageous mechanical properties (such as high mechanical strength), and relatively inexpensive production. All of these characteristics are paired with their biodegradability, renewability, and ready availability [
4,
94], enabling different types of formulations, either alone or as polymer composites [
94]. Multiple potential applications of CNCs or CNFs in the biomedical field have been explored in recent years, as extensively reviewed in [
5,
8,
13,
20,
94,
95,
96,
97,
98,
99,
100,
101,
102]. Among many biomedical applications, CNMs have been widely investigated for the delivery of hydrophilic, hydrophobic, water-soluble, and poorly water-soluble drugs, to be applied via different routes of administration [
4,
25,
95]. CNM-based systems using different carrier forms (for example, dry foams and films, aerogels, emulsions, or hydrogels) have been studied to mediate drug delivery (e.g., riboflavin, bendamustine, hydrochloride, naproxen and ibuprofen, furosemide, methotrexate, repaglinide) for oral administration [
94,
103,
104,
105,
106,
107,
108,
109]. These systems have been designed for sustained and targeted drug release, with decreased side effects and enhanced therapeutic efficacy over a prolonged period, and with the prospect of dose reduction [
104]. Applications of CNMs in bio-adhesive films have also been sought for controlled drug delivery or local drug administration, as shown for the colon-specific delivery of methotrexate [
110]. Other applications of CNMs in the biomedical field include restorative dentistry, bioprinting for tissue engineering, wound healing and tissue repair, medical implants, vascular grafts, bone tissue engineering, antimicrobial membranes, and scaffolds for human stem cell cultures, among others [
4,
5,
20,
94,
97,
111,
112,
113,
114,
115,
116,
117].
This entry is adapted from the peer-reviewed paper 10.3390/nano12193375