Polyesters are synthetic resins formed by an esterification chemical reaction with some occurring naturally [22]. In addition, there are different orientations of polyesters and, hence, different classifications. The classifications aid in determining the processing, curing kinetics, and overall applications of the resin [23]. Saturated, unsaturated polyesters (UPs) and alkyd resins are the main classifications of polyesters; however, vinyl esters are also classified as polyesters since they have a di-ester group. Vinyl esters are based on the combination of an epoxy resin with an unsaturated polymer; they have excellent properties when compared to saturated, unsaturated-type polyesters and alkyd resins [24]. Developments of non-toxic polyester-based coatings have the potential to address a wide range of pollution problems, such as air pollution and water pollution, generated during the production of conventional polyester coatings [28]. The anticorrosion properties of polyester resin modified by nanocomposites intended for steel are of interest. The goal is to produce a bio-based polyester coating with minimal cost by implementing natural products as well as modifying with nanomaterials.
1. Polyesters
Polyesters are polymers formed from a dicarboxylic acid and a diol by a polycondensation process. Polyesters were first discovered by W.H. Carother while he was working for DuPont; however, his research was incomplete. In 1941, British scientists Whinfield and Dickson discovered the synthetic polyester fiber polyethylene terephthalate (PET) [
40]. Polyester because popular in the 1970s due to its inexpensive and durable nature. Polyesters have various polymer backbones formed by esterification condensation; their applications depend on the parameters and the resulting orientation of the polymer chain [
41]. The production of polyesters includes the addition of catalysts, promoters, curing agents, binders, solvents, and pigments. The characteristics of polyesters include strong fibers, mechanically durable, hydrophobic, retention of their original form, and easy to wash and dry. The drawbacks associated with polyesters are their low melting point, moisture absorption, toxicity, gelation during polycondensation reactions, and environmental and health hazards [
42,
43]. The global market for polyesters is set to increase by an average of more than 5% annually by 2020 due to the demand for and applications of polyesters [
44].
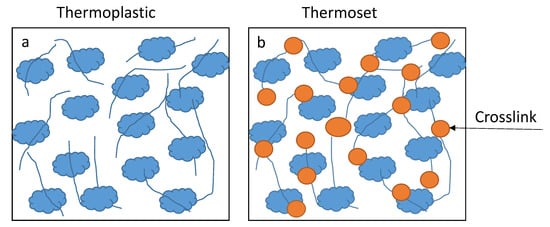
Polyesters can either be thermoset or thermoplastic polymers. Thermosets are formed by a compound with more functionality (covalent bonds) while thermoplastics are connected by weak intermolecular forces (see
Figure 4) [
45,
46]. The rigid network structure formed by thermoset polymers gives rise to various applications due to the properties they possess [
47]. Thermoplastic polyesters, PET and polybutylene terephthalate (PBT), are commercial products that are commonly used in the packaging industry [
48]. Polyesters are classified by the orientation of the polymer chain as unsaturated, saturated polyesters, alkyd resins, and vinyl esters, respectively.
Figure 4. The crosslinking process between (
a) weak intermolecular forces and (
b) strong covalent bonds [
46].
1.1. Unsaturated Polyesters
UPs are the largest group of polyesters. The backbone consist of alkyl thermosets resin [
49]. The unsaturation in these polymers is due to a double or an olefin triple bond in the hydrocarbon chain of either the acid or alcohol.
UPs are relatively inexpensive, mechanically strong, and possess tunable thermal properties. However, further modification of UPs properties with different fillers, additives, and pigments is a stimulating topic for advanced technology purposes [
53]. The polymer can be reinforced with different inorganic and organic particles for better resistance to corrosion, weather, and flame-retardant properties [
54]. The crosslinking of the polymers depends on the balance of the catalyst, inhibitors, and promoters [
55]. In particular, the extent of cure depends on the materials used in the polymerization; the impenetrable structure is formed by crosslinking with oxygen, heat, and light [
56]. The environmental aspect to reduce the amount of volatile used in UPs as crosslinkers and catalysts has been of great interest in attempts to minimize air pollution [
57]. Li et al. [
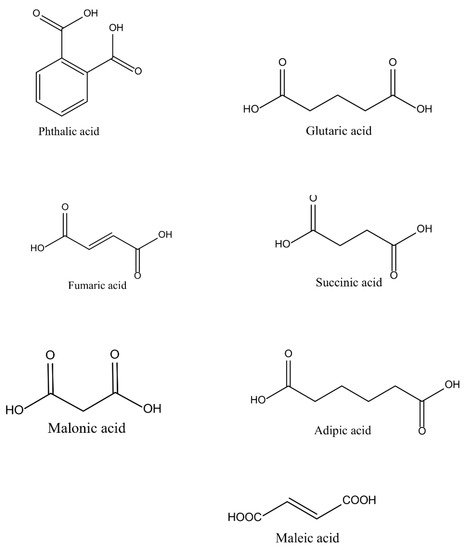
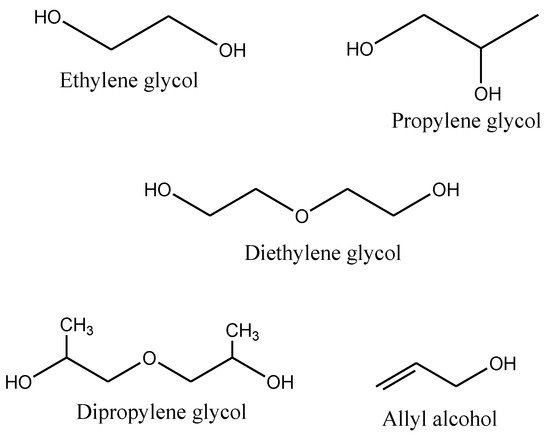
58] produced a self-curable UP, not by using crosslinking monomers but by adding vinyl groups on the end of the polymer chain to solve the problem of air pollution. The self-crosslinking reaction rate was seen to be greatly induced by the radical initiator (benzoyl peroxide) by increasing the polymerization reactivity attributed to the radical homopolymerization of the conjugated product. The raw materials commercially available for the production of UPs are petroleum based. The chemical structures of the acids and alcohols used in the synthesis of UPs are illustrated in
Figure 6 and
Figure 7, respectively. The acids include organic compounds, dicarboxylic acids, and aromatics; their applications are in the pharmaceutical, textile, and food industries [
59]. The glycols and allyl alcohol are organic compounds used as raw materials in the production of polyesters and other pharmaceutical applications [
60].
Figure 6. Some common chemical structures of acids that are used for the synthesis of UPs [
59].
Figure 7. Some common chemical structures of alcohols used for the synthesis of UPs [
60].
2.2. Saturated Polyesters
Saturated polyesters are a type of polymer without hydroxyl groups resulting from the use of excess polyol or modifying the formulation [
65]. Generally, a reaction between a dibasic acid with a diol will give a saturated polyester with an equivalent ratio poly-condensation process. They are linear and possess structures similar to simple polymers. Depending on the degree of polymerization, they are either solid or highly viscous [
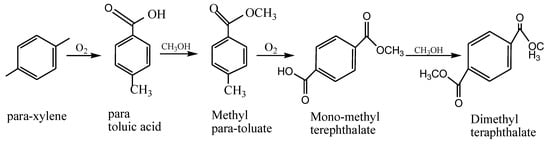
66]. The production of terephthalate follows the scheme in
Figure 8.
Figure 8. Reaction for the synthesis of a dibasic acid terephthalate.
Saturated polyesters have various properties, including high resistance to abrasion, bacteria, resistance to chemical attack, high strength, and flexibility, and possess good dielectric properties [
69]. Their hydrophilic nature is responsible for the poor mechanical properties. Environments with high temperatures account for the decreased structural properties of saturated polyesters [
70]. Lee and colleagues [
71] investigated the synthesis of four types of flexible polyester resins with urethane polyol of polycarbonatediol (PCDL) isocyanate and carboxylic-terminated polyester pre-polymer. It was found that increasing the concentration of the polyol by approximately 300 ppm increased the flexibility of the polymer observed from the Fourier transform infrared (FTIR) spectrum detected at 1240 and 1760 cm
−1 and the increased intensity of these bands. This was attributed to the soft segment and highly rubbery nature of the urethane polyol monomer. The polymer cures by free radical reactions, which are related to aging and oxidation.
2.3. Alkyd Resins
A reaction between a polyhydric alcohol and a fatty acid followed by a reaction with a dibasic acid gives an alkyd resin [
75]. Glycerol is the most common type of polyalcohol used and phthalic anhydride is used as a polybasic acid. Alkyds can be modified by substituting a polybasic acid with a monobasic acid [
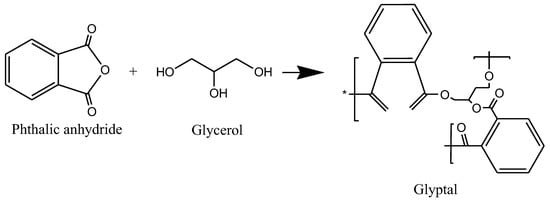
76]. The polycondensation reaction between glycerol and phthalic anhydride giving polyester glyptal alkyd resin is illustrated in
Figure 10.
Figure 10. The reaction of glycerol with phthalic anhydride gives glyptal alkyd resin.
Other polybasic acids used include maleic anhydride, fumaric acid, and isophthalic acid and the polyols used include ethylene glycol, trimethyloethane, and neopentyl glycol, among many others [
77]. The properties of alkyds include compatibility with other coating polymers [
78]. Alkyd resins have applications in paints, thermosetting polymers, and the printing industry [
79].
2.4. Vinyl Esters
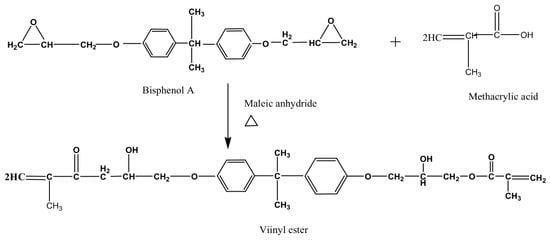
Vinyl esters are polymers derived from vinyl alcohols [
80]. The reaction of glycidyl acrylate and glycidyl methacrylate with bisphenol A through an esterification process is one example of forming the vinyl esters [
81]. The functionalities are based on the combination of epoxy resin with an unsaturated polymer. There are very reactive with polar groups that give the excellent properties associated with vinyl esters. Vinyl esters are easy to handle at room temperature, which gives greater control over the curing rate [
82]. However, the short shelf life is a drawback for quality control. A reaction to form a vinyl ester resin is illustrated in
Figure 11.
Figure 11. The reaction of bisphenol A and methacrylic acid with maleic anhydride as a catalyst at a temperature between 90–120 °C to form a vinyl ester resin.
Vinyl esters are sometimes classified as a polyester but they are di-esters due to the backbone of the chain that contains a link of ether groups [
83]. The crosslinking is similar to that of UPs; however, the physical properties are superior due to the presence of olefin groups in the polymer chain [
84]. The excellent properties of vinyl esters include good mechanical strength and resistance to chemical, solvent, and corrosion attacks [
85]. Zhang et al. [
86] studied the effect of isothermal temperature on the curing extent, gel time, rheology, and the mechanical properties of vinyl ester resin. The researchers found that the extent of cure depends on the isothermal temperature, while the tensile strength (higher elongation) increased with the increase in crosslinking density. The shear storage and loss modulus at the gel point were also observed to decrease with increases in both the isothermal temperature and the heating rate. The formation of micro-gels during the gelation process was the reason for this observation.
2. Polyester-Based Coatings
Dai et al. [
128] synthesized bio-based unsaturated polyester resins and their application in water-based coatings cured by UV radiation applied on a tin plate substrate. Three polyesters were formed with good properties and the reduction of the emission of volatile organic compounds was achieved in this production. Lee and his colleagues synthesized and characterized the polyester-based nanocomposites coatings for application in the automobile for pre-coated surfaces [
129]. The polyethylene (PE) polymer was polymerized with various clay nanoparticles to form a composite; the pre-coated metal improve productivity. Good dispersion of the organically modified MMT in the PE polymer improved the mechanical, viscoelastic, and anticorrosion properties. The application using roll coating processes instead of a wet coating process decreased the problem of air pollution that occurs during evaporation. However, polyester-based compounds still have many drawbacks, such as the generation of volatile organic compounds, high labor intensity, emission of harmful by-products, and high costs. Atta et al. [
130] studied unsaturated polyester resin based on rosin maleic anhydride adduct for the corrosion protection of steel. The findings of the study revealed that unsaturated polyesters based on the rosin adduct can be used for corrosion protection of steel with improved properties.
Corrosion protection of carbon steel oil pipelines by unsaturated polyester/clay composite coatings was also investigated by Ramesh et al. [
131]. The results indicated that the protected film surface stability and its resistance to dissolution were the crucial parameters in corrosion protection when applied to carbon steel. In a different study, Ramesh and his colleagues investigated silicone polyester blended coatings for corrosion protection. The hybrid system showed to have protection efficiency for more than 30 days, as well as good thermal properties. In references [
132], the synthesis and characterization of hyper-branched polyester-urethane-urea (k10-clay) hybrid coatings was reported. The formation of 63% condensation product was achieved while the hydrophilicity of the hybrid coating is increased, attributed to the direct proportion increase in the clay and urea ratio. A similar study by Piazza et al. [
133] showed that polyester-based powder coatings with montmorillonite (MMT) nanoparticles improved the anticorrosive properties when applied to carbon steel substrates. However, the thermal stability of the coating was decreased when higher concentrations of MMT were used, which was attributed to the oversaturation of the clay in the polymer matrix. Chen et al. [
134] investigated the in situ polymerization and characterization of polyester-based polyurethane/nanosilica composite. It was reported that the chemical interaction in the polymerization improved the mechanical properties due to the nanoparticles being located in the interface and surface of the polymer when applied to tinned iron. The corrosion performance improvements of hot-dipped galvanized steel by electrodeposition of epoxy resin-ester modified BTSE (bis-tri-ethoxy-silyl-ethane) coatings were reported by Xue and colleagues [
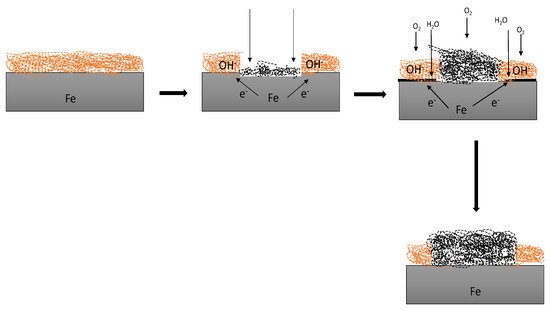
135]. They showed that coating using electrodeposition achieved better corrosion protection than immersion coating, which was attributed to the uniformity and non-porosity of the films. The application process of coating affects the overall performance of the coating. The excellent properties of polyesters will reduce the need for re-application processes and replacement of damaged structures affected by corrosion as depicted in
Figure 14.
Figure 14. The degradation mechanism of a coated metal exposed to NaCl for 30 days from the initial state, when there was an attack from the atmosphere and the resultant product with rust.
Vinyl esters are mostly used for excellent mechanical and adherent properties and resistance to corrosion attack [
136]. However, the short shelf life is a drawback for quality control. Gopi et al. [
137] synthesized and characterized the corrosion protection of poly (N-vinyl carbazole-co-glycidyl methacrylate) coatings applied on low nickel stainless steel. The results showed that the ratio of the copolymer improved the corrosion protection in 0.5 M H
2SO
4 medium by the formation of a barrier effect from the polymer layer. The protection efficiency is highly dependent on the composition of the copolymer. In another study, Hollamby and colleagues investigated the hybrid polyester coating incorporating functionalized mesoporous carries for the holistic protection of galvanized steel surfaces [
138]. A 96% corrosion efficiency was achieved, attributed to the use of a dense polymer and mesoporous silica nanoparticles.
4. Applications
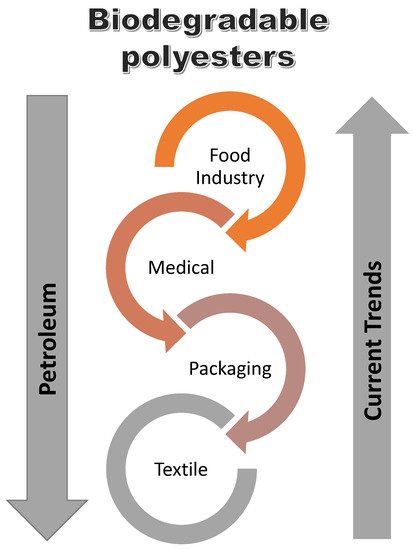
The applications of polyesters and vinyl esters depend on the properties of the resin. Different industries apply polyester and vinyl ester resin due to their excellent properties as already discussed. The different industries are outlined in Table 2 and the potential growth in the application of biodegradable polymers is depicted in Figure 15.
Figure 15. Current trends in the use of biodegradable polyester vs petroleum-based coatings [
147].
Table 2. Other applications of polyesters.
| Industry |
Type of Polyester |
Properties |
Reference |
| Transportation |
|
-
High resistance to temperature
-
High strength
-
Hardness
-
Excellent sliding properties
-
Electric insulation
-
High resistance to abrasion and modulus
|
[148,149,150] |
| Electrical |
|
|
[151,152,153] |
| Industrial applications |
|
|
[154,155,156,157,158] |
| Construction |
Ethylene tetrafluoroethylene (ETFE) |
|
[159,160] |
This entry is adapted from the peer-reviewed paper 10.3390/polym14163413