Degenerative cervical myelopathy (DCM) manifests as the primary cause of spinal cord dysfunction and is non-traumatic, chronic and progressive in nature. Decompressive surgery is typically utilised to halt further disability and neurological dysfunction and as such, early diagnosis and assessment is a vital means of slowing the disease process. Currently, there exists a plethora of methods for diagnosing DCM, each with their own unique limitations. This review aims to outline such limitations of current diagnostic techniques and present some novel quantitative MRI (qMRI) techniques for assessing spinal cord integrity in DCM.
- cervical spine
- degenerative cervical myelopathy (DCM)
- cervical spondylotic myelopathy (CSM)
- spinal cord compression
- spinal cord integrity
- spinal cord injury
- neurodegeneration
- diagnosis
1. Epidemiology
2. Natural History
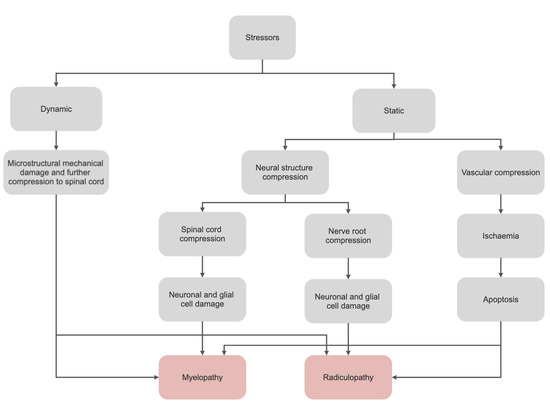
3. Current Diagnostic Options and Limitations
3.1. Clinical
| Presenting Symptoms | Physical Signs | |
|---|---|---|
| Neck |
|
|
| Upper Limb |
|
|
| Lower Limb |
|
|
| Urinary/defecatory |
|
|
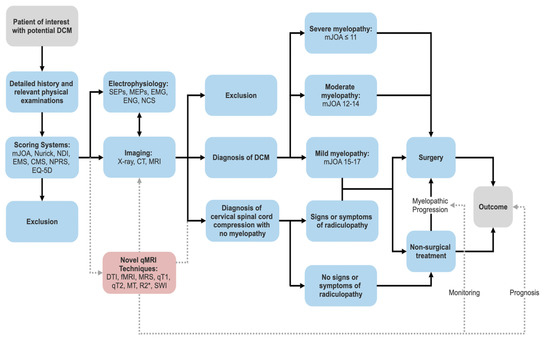
3.2. Scoring Systems
Clinicians utilise scoring systems to categories the functional impairment of various conditions. Whilst different classifications may arise, there typically exists one standardised system for publications and treatments. This is not true for DCM; whereby different systems are utilised based upon preference. A 2016 systematic-review revealed that reported outcomes varied widely between studies of DCM [45]. Table 2 details the current most common classification systems, their benefits and limitations. The mJOA scale followed by the Nurick Grading system are the current most widely adopted measure for DCM patients [46].
However, the limitations covered in Table 2, are particularly problematic in mild DCM whereby strong floor and ceiling effects[e] in these scales inhibit ascertainment of more subtle neurological changes that provide information for decision-making in surgery [18]. As such, there exists the need to develop both a standardised scoring system and more sensitive and objective outcome instruments to enable more effective clinical assessment and efficient synthesis of research.
Table 2. Common classification systems used for DCM [17,47–56].
|
System |
Description |
Benefits |
Limitations |
|
mJOA scale |
- 0–18. A lower score indicates a more severe deficit. - Normal: 18 - Upper-extremity function (5) - Lower-extremity function (7) - Sensory function (3) - Bladder function (3) |
- Good for assessing outcomes (post-operative). - Specific to DCM - Responsive to change - Commonly used in research - Clinician administered |
- No economic factors taken into consideration. - Poor sensitivity - Ceiling effect: hard to detect minor improvements in patients with mild disease - Modest intra-rater and inter-rater reliability (the minimum detectable change is two points). - Four categories are not equally weighted. |
|
Nurick scale |
- 0–5. A higher grade indicates a more severe deficit. - Myelopathy (6 points) |
- Good for evaluating economic situation in conjunction with gait function. - Specific to DCM - Commonly used in research - Consists of both impairment and disability components |
- Low sensitivity - Poor responsiveness with limited ability to detect change. - Less accurate for post-operative grading. - Cannot detect upper extremity dysfunction. |
|
NDI |
- 0–50. A higher grade indicates a more severe disability. - Neck disability (10 subsections) - 0 = no disability - Consists of: Pain intensity, personal care, lifting, reading, headaches, concentration, work, driving, sleeping, recreation |
- Fair interobserver reliability in patients that have cervical radiculopathy - Responsive to change - Incorporates various activities from daily living |
- Validity and reliability only evaluated in neck pain patients and cervical radiculopathy patients - Subjective; patient reported - Not specific to DCM
|
|
EMS |
- 5–18. A lower score indicates a more severe deficit. - Normal: 17+ |
- Good at evaluating clinical state and level of severity. - Better sensitivity towards functional deficits (as it assesses coordination and proprioception) |
- Not commonly used in research |
|
CMS |
- Upper/lower extremities are analysed separately 0–5 each. - A higher grade indicates a more severe deficit. |
- Good for evaluating upper/lower extremity function as they are elicited separately. - Good at evaluating clinical state and level of severity. |
- No economic factors taken into consideration. |
|
NPRS |
- 0–10. A higher score indicates a more severe disability |
- Simplicity and reproducibility - Sensitive to small changes - Valid |
- Not specific to DCM - Subjective - Suffers from the ceiling effect |
|
EQ-5D |
- A standardised measure of health status looking into mobility, self-care, activities of daily living, pain/discomfort, anxiety/depression. - Not measured on a numbered scale |
- Ease of completion - Sensitive to change - Useful for looking into health economic evaluations |
- Emotions and mood are limited to anxiety and depression - Quite global in nature - Overlooks some dimensions of quality of life (spiritual, social) - Does not include cognition - Not specific to DCM |
|
Additional scales that provide useful information in the context of DCM include the Myelopathy Disability Index, QuickDASH (assesses arm, shoulder and hand disability), the 30-Metre-Walk test, the Berg Balance Scale, GAITRite (a temporospatial gait analysis) and the Graded Redefined Assessment of Strength Sensibility and Prehension Myelopathy (GRASSP-M). |
|||
Abbreviations: CMS, Cervical Myelopathy Scale; DCM, degenerative cervical myelopathy; EMS, European Myelopathy Scale; mJOA, modified Japanese Orthopaedic Association; NDI, Neck Disability Index; NPRS, Numeric Pain Rating Scale.
3.3. Conventional MRI
Conventional MRI is the primary modality utilised for imaging in DCM as it enables high-resolution depiction of neural structures, bone and ligaments that are difficult to visualise in other scans [57]. Conventional MRI (such as T1-weighted and T2-weighted imaging) can characterise the degree and nature of degeneration (i.e., OPLL, spondylosis, disc herniation, hypertrophy of ligamentum flavum), identify spinal-cord compression, highlight changes in spinal-canal diameter, and detect changes in signal intensity [58–60]. MRI can also assist in ruling-out resembling differentials or other causes of myelopathy such as a tumour syringomyelia or demyelinating plaques [2,38,42]. CT myelography should be utilised in situations of MRI contraindication [61].
Identifying spinal-cord compression plays a pivotal role in treatment selection and outcome prediction and thus should be the foremost investigation. It is typically described based on the number of compression sites [30], appearance [32,62–65] or ratio between the anteroposterior diameter and the transverse diameter (CR = Compression Ratio) [66,67]. A maximum spinal-cord compression (MSCC) index has also been developed by Fehlings et al. as a measurement of spinal-cord compression [68]. The primary object of these methods is to determine severity of spinal-cord compression.
Measurements of the anterior-posterior diameter at the region of interest (ROI) can be undertaken to evaluate the degree of spinal-stenosis [30]. Similar to MSCC, Fehlings et al. have developed a protocol to assess the maximum canal compromise (MCC) post-traumatic cervical spine-injury [69]. This has been additionally utilised for degenerative conditions and functions by calculating the canal size at the ROI and analysing it in conjunction to the average canal size for levels above and below. Multi-level signal-intensity changes are suggestive of necrosis or cavitation in the spinal cord and lend to poorer surgical outcomes [70–73]. T2-hyperintensity in conjunction with T1-hypointensity is associated with greater clinical deterioration when compared to T2-hyperintensity alone due to signal changes in T1-weighted images indicative of more permanent insult [70,74–77].
Limitations: Findings on conventional MRI do not typically correlate well with the variable clinical presentations of DCM [42]. Although spinal-cord compression is a sensitive marker of myelopathy [78], approximately 5% of asymptomatic patients also present with it [42], thereby limiting its specificity. The supine patient positioning in conventional MRI hinders its utility in assessing alignment, providing only a superficial assessment for situations in which upright films are not available [79]. Conventional MRI is intrinsically limited in its capability to characterize tissue injury in the spinal-cord because of the lack of specificity in T1/T2WI signal-change and cannot highlight specific pathophysiological processes at a cellular level (demyelination, axonal loss, inflammation, oedema, gliosis and apoptosis) [57]. It also is not a good predictor of neurologic function before/after surgical intervention and has low sensitivity for structural spinal cord change in cervical myelopathy [57,70,80–83].
3.4. Plain Radiographs and Computed Tomography (CT)
Computed Tomography is useful for the study of bone anatomy and can aid in cases where spinal-fusion is being considered as a treatment. In cases where MRI is contraindicated (such as the presence of pacemakers or other internal metallic objects), CT is a valuable imaging alternative. Plain radiographs can provide useful information about spinal-canal stenosis, degenerating discs, degenerating joints, OPLL, vertebrae fusion, cervical-spine alignment and subluxation [2,38,84,85]. This can reveal scoliosis and loss of physiological cervical-lordosis and kyphosis. Lateral-films in cervical-flexion and extensions are utilised to evaluate instability of the cervical-spine. DCM patients frequently showcase increased C2-C7 Cobb angles, upper T1 slopes, lower C7 slopes and upper C7 slopes [86].
Limitations: Computed tomography suffers the same inability to characterise tissue injury that conventional MRI does [57]. In addition, a 2017 systematic-review found that the overall strength of evidence regarding the predictive value that CT parameters have for the clinical presentation or outcome of DCM is low [87]. There is also the issue of radiation exposure. Overall CT and plain radiographs play a more complementary role in DCM diagnosis, acting as an alternative to MRI and aiding in surgical-planning [88].
3.5. Electrophysiology
Several studies have indicated good correlation between electrophysiology and the severity of myelopathy, presenting it as a reliable predictor of surgical-outcomes [89]. Somatosensory evoked-potentials (SEPs) and motor evoked-potentials (MEPs) can be, respectively, utilised to detect central sensory conduction impairment and prolonged motor latency in DCM [2,89,90]. They are also useful in detecting subclinical degenerative spinal-cord compression in asymptomatic patients and are thus useful in early identification of patients likely to develop myelopathy [91–94]. Feng et al. reported a correlation between the SEP and a declining mJOA (a more severe deficit) in an investigation of progressive myelopathy [95]. Needle electromyography (EMG) is a highly sensitive indicator of anterior horn cells damage, which occurs due to compression and ischemia in DCM [96]. Nerve-conduction studies can also be used to rule out peripheral neuropathy and nerve-entrapment [2]. These techniques also allow other neuromuscular diseases that can mimic DCM to be ruled out (motor neurone disease, ALS) [97,98]. Apart from aiding in diagnosis and preoperative evaluation, electrophysiology facilitates longitudinal assessment. Capone et al. found that a decrease in central-motor conduction time for the tibialis-anterior muscle correlated with an increased mJOA score post-surgery. It therefore concluded that the beneficial effects of spinal-cord surgery could be detected with MEP, making it a useful tool in determining efficacy of post-operative rehabilitation [99].
Limitations: Electrophysiology provides no anatomical information and thus cannot determine the exact location of the lesion [100]. Although some evidence exists to justify the effectiveness of electrophysiology in predicting operative outcomes, the area remains to be better defined. A systematic review found a decrease in electrophysiology publications compared with other domains of DCM, suggesting a declining interest in this area [101]. Additional studies would be required before it can be universally recommended.
4. Novel qMRI Modalities and Parameters
[70,103,104,105,106,107,110,111,112,113,114,115,116,117,118,119,120].
|
Sequence |
Function |
Quantitative Metrics |
|
|
Quantitative T1/T2 Mapping |
Calculates the T1/T2 time of certain tissues and displays them on a parametric map. Reveals information about microstructural changes related to water, lipid, protein and iron content of tissues. |
T1/T2 relaxation time |
|
|
DWI |
DTI |
Estimates the integrity of tissue microstructure through the modelling of water diffusion within the tissue. |
FA [f], ADC, MD [g] |
|
DTT |
Tracks nerve fibres based on their FA values and can be elicited when fibres become interrupted, distorted or disorientated depending on the severity of spinal compression. |
Volume and number of fibres |
|
|
DBSI |
Quantifies axonal injury, inflammation and demyelination in DCM |
Axonal injury, inflammation, demyelination. |
|
|
fMRI (BOLD) |
Measures neuronal activity through associated changes detected in blood flow |
FC, VOA |
|
|
MT |
Provides information on the spinal cord structural integrity and derive information regarding myelination status |
MTR |
|
|
MRS |
Sensitive to metabolic changes that occur in pathology, reflecting important underlying biological mechanisms |
Metabolite concentrations |
|
|
T2*-weighted imaging |
Quantifies observable or effective T2 and is utilised to detect deoxyhaemoglobin, hemosiderin or methemoglobin in tissues and lesions. |
R2* (=1/T2*) |
|
|
SWI/QSM |
Sensitive to compounds that distort the magnetic field and alter phase of tissue and is therefore commonly used to detect blood products/haemorrhage and calcium |
Tissue susceptibility |
|
Abbreviations: ADC, apparent diffusion coefficient; BOLD, blood oxygen level dependent; DBSI, diffusion basis spectrum imaging; DCM, degenerative cervical myelopathy; DTI, diffusion tensor imaging; DTT, diffusion tensor tractography; DWI, diffusion weighted imaging; FA, fractional anisotropy; FC, functional connectivity; fMRI, functional magnetic resonance imaging; MD, mean diffusivity; MRS, magnetic resonance spectroscopy; MT, magnetisation transfer; MTR, magnetisation transfer ratio; QSM, quantitative susceptibility mapping; SWI, susceptibility weighted imaging; VOA, volume of activation. [f] Fractional anisotropy (FA): Water molecules diffuse differently along tissues depending on its type, integrity, architecture, and presence of barriers. Fractional anisotropy is a value between 0 and 1 which indicates the degree to which diffusion of water is limited to one axis; [g] Apparent diffusion coefficient (ADC)/mean diffusivity (MD): measures of the average magnitude of water diffusion within a tissue.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10102621
