Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The imperative to substantially expand the world’s seaweed aquaculture supply is well established in published literature and has the strong backing of virtually all global non-government organizations (NGOs). The expansion of seaweed farming is recognised as one of the best approaches to realising many of the sustainable development goals of the United Nations.
- seaweed
- infrastructure
- longline
- offshore wind
1. Introduction
The demand for seaweed products in a low-carbon world is extensive and growing [1][2]. Seaweed as a traditional food source (used in salads, soups, sushi wraps, etc.) provides for a low-calorie diet that supplies vitamins A, B, C, and E, dietary fibre, omega-3 fatty acids, essential amino acids, and has been shown to improve digestive health, reduce the risk of colorectal cancer, and reduce obesity [3]. Seaweed-derived hydrocolloids are used in many food products, such as salad dressings, ice cream, and beverages, and account for about 40% of the global hydrocolloids market [4]. Other uses include fertilisers, cosmetics, nutraceuticals, pharmaceuticals, and the emerging markets for bio-plastics, fabrics, bio-fuels, bio-char, and potentially carbon sequestration. Seaweed also plays an important role in the aquatic ecosystem, providing eutrophication mitigation, shoreline protection, and habitat for aquatic organisms as nursery grounds [1].
In response to concerns about greenhouse gas emissions the beef cattle market is being challenged by changing dietary patterns and the growth of lab-grown meat and vegan substitutes. The inclusion of small quantities of specific seaweed species as feed additives has been shown to substantially reduce enteric methane production in beef and dairy cattle. For example, the inclusion of the red seaweed Asparagopsis Taxiformis at a dietary dry matter level of just 0.2% yielded a methane reduction of 98% relative to a controlled beef steer group [5]. In another study, the inclusion of a closely related species Asparagopsis Armata in Holstein dairy cattle at a rate of 1% dry matter yielded a methane reduction of 67.2% [6]. Notably, these and other studies on seaweed feed supplementation reported simultaneous improvements in feed efficiency for beef cattle and milk production [7][8].
The overwhelming majority of current seaweed farming production is in nearshore-sheltered and intertidal shallow waters where simple systems combine with low labour costs for their feasibility. However, existing nearshore-sheltered farming practice is facing a number of challenges to its continued growth. Many nearshore-sheltered coastal sites are in direct competition with other uses in marine spatial planning—namely tourism, shipping, and fishing [9]. In many places, particularly in tropical and lower latitudes, rising upper pelagic ocean temperatures are limiting the productivity of seaweed farming [10]. There are also a number of environmental risks in nearshore-sheltered farming including the slowing of water flows in protected areas, and the spread of disease and parasites under some circumstances. Farming offshore or in exposed coastal waters would avoid the trampling and shading of natural macroalgal beds and seagrasses, reduce the incidence of herbivorous fish grazing, fouling, and epiphytic growth, and help to reduce ocean acidification [1][11][12][13].
2. Traditional Farming Methods
2.1. Background and Seaweed Species Classification
As with any form of aquaculture the infrastructure and cultivation methods used are highly dependent on the seaweed species and their ideal growing environment. The most commonly farmed species and their uses and basic infrastructure are summarised in Table 1. The simplest systems can often provide inspiration for further development. Examples of bamboo rafts and twine-growing frames, tube nets, simple monolines, buoy-supported longline systems, and staked rope bottom culture are illustrated in Figure 1. The most basic and labour intense systems, such as the bamboo rafts, and staked rope bottom cultures (typically used in the tropics in small-scale farming of red seaweed) rely on manual fragmentation and seedling propagule attachment to seeding ropes [14][15]. Simple tube-net systems are used to give improved resistance to nearshore wave damage.

Figure 1. Simple seaweed systems—raft, monoline, tube nets-images from https://www.slideshare.net/zoysa89/sea-weed-farmingsouth-east-asia (accessed on 22 September 2022), floating longlines (bottom left) [16], staked rope bottom culture (bottom right) [16].
Table 1. Seaweed species use and infrastructure.
| Species Group | Seaweed Colour | Biomass Load per Metre (Scale 1–3) | Tonnes (Wet) Annual Cultivation [1] | % World Market [1] | Buoyancy | Region | Applications | Cultivation Method(s) | Hydrodynamic Suitability (Scale 1–3) |
|---|---|---|---|---|---|---|---|---|---|
| Laminaria/Saccharina | Brown | 2—Moderate | 12,273,748 | 35.4% | Neutral/slightly negative | Temperate | Human consumption; Raw material for alginate, mannitol, iodine, Abalone feed | Longlines | 2—Moderate, grows in exposed water |
| Undaria (wakame) | Brown | 2—Moderate | 2,563,582 | 7.4% | Negative | Temperate | Sea mustard, Abalone Feed | Longlines | 1–2—Low–moderate, grows in exposed waters |
| Macrocystis pyrifera | Brown | 3—High | 2 | 0.0% | Positive | Temperate | Food and Cosmetic Products, Animal Feed | Longlines | 2—Moderate, grows in exposed waters |
| Sargassum | Brown | 3—High | 304,000 | 0.9% | Positive | Tropical | Food and Cosmetic Products, Animal Feed | Longlines | 2—Moderate, grows in exposed waters |
| Alaria esculenta | Brown | 105 | 0.0% | Temperate | Animal feed | Longlines | 2—Moderate, grows in exposed waters | ||
| Eckolonia, Lessonia | Brown | 2—Moderate | - | - | Negative | Temperate | Human consumption, fertiliser, animal feed (e.g., livestock, aquaculture), nutraceuticals, and biopolymers and bioplastics | Longlines | 2—Moderate, grows in exposed water |
| Durvillaea | Brown | 3—High | - | - | Negative | Temperate | Alginate industry, fertiliser | Longlines | 3—High, grows in very exposed waters |
| Kappaphycus/Eucheuma | Red | 2—Moderate | 11,622,213 | 33.5% | Negative | Tropical | For carrageenan extraction | Longlines/nets | 1–2—Low–moderate, grows in open water |
| Gracilaria | Red | 1—Low | 3,639,833 | 10.5% | Negative | Tropical | Feed for abalone; For agar extraction; Bioremediation | Longlines/nets | 1–2—Low–moderate, grows in exposed water |
| Porphyra | Red | 1—Low | 2,984,123 | 8.6% | - | Temperate | Food wrap | Nets | 1—Low–delicate |
| Asparagopsis | Red | 1—Low | - | - | - | Tropical/warm temperate | Animal feed | Longlines, net tubes | 1—Low–delicate, unsuitable for open waters |
| All Green | Green | 1—Low | 14,019 | 0.0% | Tropical/temperate | Human consumption | Land-based facilities | 1—Low–delicate | |
| TOTAL | 34,679,134 |
With a few notable exceptions, such as free-floating Sargassum spp., almost all wild-found and farmed seaweed rely on seaweed attaching to some structure through their root-like “holdfast” organ. Given adequate sunlight for photosynthesis, nutrient availability, salinity, temperature range, oxygen, and carbon dioxide, seaweed propagules can grow regardless of how deep the sea beneath is.
Depending on the fragility of the species, the water speed, through wave action, currents, or tides, needs to be kept within upper bounds to avoid damage and lower bounds for optimal nutrient uptake and growth [14][17][18].
It has been shown that increased tension in sub-surface culture lines increases water flow and, hence, the growth of some kelp species in low current and surface wave conditions [17][18]. However, in moderate to higher energy environments though, this increased tension reduces the ability of the culture line to dampen wave and current energy risking seaweed holdfast detachment and higher mooring loads. A further consideration for system design is the buoyancy of different species. Positively buoyant species such as Macrocystis spp. that develop pneumatocysts (air-sacs) grow up from their holdfast, whereas most other species are typically slightly negatively buoyant. This variation in species buoyancy, size, and drag inevitably means that infrastructure systems need to be customised and adapted into solutions to suit different groups sharing similar characteristics.
2.2. Cultivation Systems
Larger operators in more temperate regions typically automate the seeding of longlines in laboratory based hatcheries [19]. By adjusting light and temperature conditions in spawning tanks seaweed spores are induced, settled, and grafted onto coiled longlines. These are subsequently deployed at a suitable depth by using float buoys and concrete moorings in the grow-out phase. Depending on the species, these longlines are either harvested completely and replaced with new freshly seeded longlines or trimmed every few weeks and allowed to regrow multiple times throughout a growing season or for several years in some cases—a technique known as multiple partial harvesting. An alternate variation uses fine strings to seed spores, which are then in turn bound to a larger culture rope, as shown in Figure 2 [17]. Depending on the species and its buoyancy, line systems must be devised that control the average depth of the seaweed for growth optimisation, minimise damage to due wave and current action, and allow for efficient harvesting. The major variations of traditional culture line systems used for most neutral or negatively buoyant species are categorised as horizontal longlines, vertical lines, or garland lines are shown in Figure 3 [20].
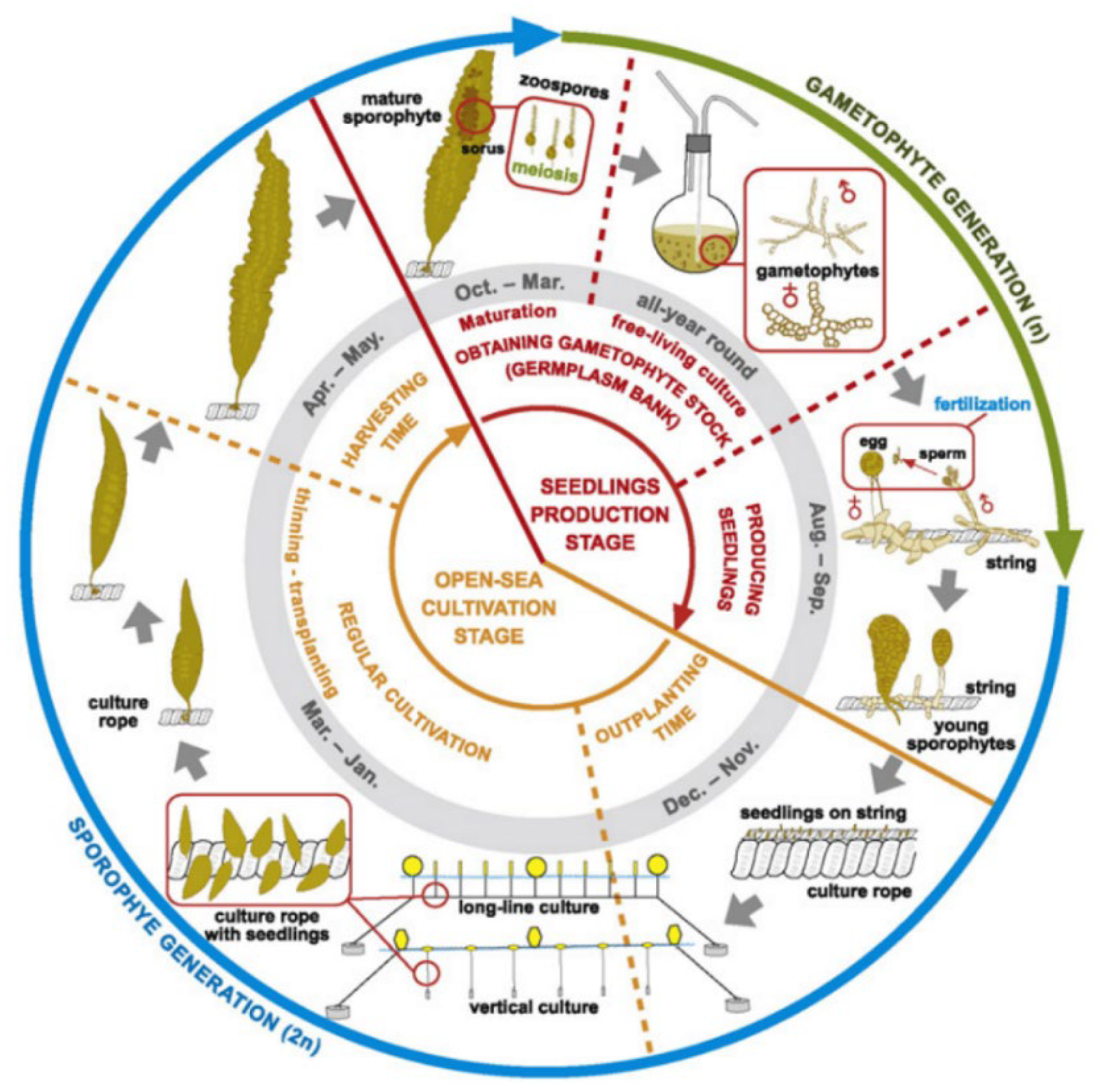
Figure 2. Typical cultivation lifecycle in the northern hemisphere of Saccharina latissimi using seeded strings. Reproduced with Permission [17].
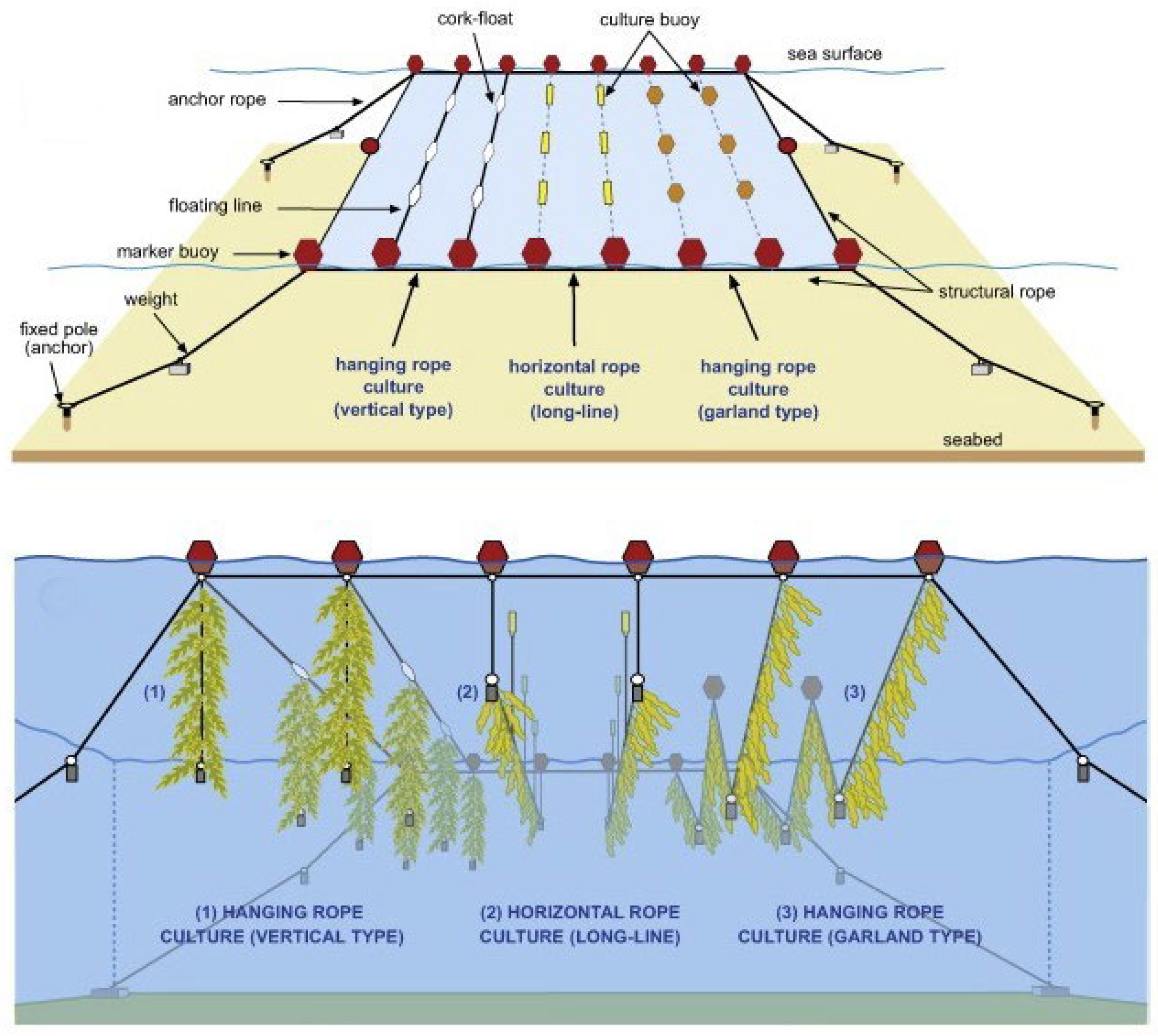
Figure 3. Variations on traditional culture rope systems. Reproduced with Permission [17].
For some species, such as many of the green seaweeds, their weak resistance to waves and currents makes them unsuitable for farming in open waters. These are typically grown in tank-based land facilities where water temperature and water biochemistry can be easily controlled. However, the higher costs inherent in these systems restrict this market to high-value (food uses) applications. Examples of land-based seaweed farming are shown in Figure 4.

Figure 4. (a) Farming Ulva and Gracilaria in Israel (https://seakura.co.il/en/ (accessed on 22 September 2022)), (b) farming Umbibudo sea grapes in Vietnam (http://www.dtvietnam.com/# (accessed on 22 September 2022)).
This entry is adapted from the peer-reviewed paper 10.3390/jmse10101447
References
- FAO. Seaweeds and Microalgae: An Overview for Unlocking Their Potential in Global Aquaculture Development; FAO: Rome, Italy, 2021.
- White, W.L.; Wilson, P. World seaweed utilization. In Seaweed Sustainability: Food and Non-Food Applications; Academic Press: Cambridge, MA, USA, 2015; pp. 7–25.
- Rajapakse, N.; Kim, S.K. Nutritional and digestive health benefits of seaweed. Adv. Food Nutr. Res. 2011, 64, 17–28.
- FAO. The global status of seaweed production, trade and utilization. FAO Globefish Res. Program. 2018, 124, 120.
- Kinley, R.D.; Martinez-Fernandez, G.; Matthews, M.K.; de Nys, R.; Magnusson, M.; Tomkins, N.W. Mitigating the carbon footprint and improving productivity of ruminant livestock agriculture using a red seaweed. J. Clean. Prod. 2020, 259, 120836.
- Roque, B.M.; Salwen, J.K.; Kinley, R.; Kebreab, E. Inclusion of Asparagopsis armata in lactating dairy cows’ diet reduces enteric methane emission by over 50 percent. J. Clean. Prod. 2019, 234, 132–138.
- Vijn, S.; Compart, D.P.; Dutta, N.; Foukis, A.; Hess, M.; Hristov, A.N.; Kalscheur, K.F.; Kebreab, E.; Nuzhdin, S.V.; Price, N.N.; et al. Key Considerations for the Use of Seaweed to Reduce Enteric Methane Emissions From Cattle. Front. Vet. Sci. 2020, 7, 597430.
- Costa, M.; Cardoso, C.; Afonso, C.; Bandarra, N.M.; Prates, J.A.M. Current knowledge and future perspectives of the use of seaweeds for livestock production and meat quality: A systematic review. J. Anim. Physiol. Anim. Nutr. 2021, 105, 1075–1102.
- Buck, B.H.; Troell, M.F.; Krause, G.; Angel, D.L.; Grote, B.; Chopin, T. State of the art and challenges for offshore Integrated multi-trophic aquaculture (IMTA). Front. Mar. Sci. 2018, 5, 165.
- Duarte, C.M.; Bruhn, A.; Krause-Jensen, D. A seaweed aquaculture imperative to meet global sustainability targets. Nat. Sustain. 2022, 5, 185–193.
- Unsworth, R.K.F.; Ambo-Rappe, R.; Jones, B.L.; La Nafie, Y.A.; Irawan, A.; Hernawan, U.E.; Moore, A.M.; Cullen-Unsworth, L.C. Indonesia’s globally significant seagrass meadows are under widespread threat. Sci. Total Environ. 2018, 634, 279–286.
- Bak, U.G.; Gregersen, Ó.; Infante, J. Technical challenges for offshore cultivation of kelp species: Lessons learned and future directions. Bot. Mar. 2020, 63, 341–353.
- Clements, J.C.; Chopin, T. Ocean acidification and marine aquaculture in North America: Potential impacts and mitigation strategies. Rev. Aquac. 2017, 9, 326–341.
- Valderrama, D.; Cai, J.; Hishamunda, N.; Ridler, N.; Neish, I.C.; Hurtado, A.Q.; Msuya, F.E.; Krishnan, M.; Narayanakumar, R.; Kronen, M.; et al. The Economics of Kappaphycus Seaweed Cultivation in Developing Countries: A Comparative Analysis of Farming Systems. Aquac. Econ. Manag. 2015, 19, 251–277.
- FAO. Social and Economic Dimensions of Carrageenan Seaweed Farming. 2013. Available online: https://www.fao.org/in-action/globefish/publications/details-publication/en/c/338356/ (accessed on 22 September 2022).
- Bjerregaard, R.; Valderrama, D.; Radulovich, R.; Diana, J.; Capron, M.; Mckinnie, C.A.; Cedric, M.; Hopkins, K.; Yarish, C.; Goudey, C.; et al. Seaweed Aquaculture for Food Security, Income Generation and Environmental Health in Tropical Developing Countries; World Bank Group: Washington, DC, USA, 2016.
- Peteiro, C.; Sánchez, N.; Martínez, B. Mariculture of the Asian kelp Undaria pinnatifida and the native kelp Saccharina latissima along the Atlantic coast of Southern Europe: An overview. Algal Res. 2016, 15, 9–23.
- Neushul, M.; Benson, J.; Harger, B.W.W.; Charters, A.C. Macroalgal farming in the sea: Water motion and nitrate uptake. J. Appl. Phycol. 1992, 4, 255–265.
- Hurd, C.L.; Harrison, P.J.; Bischof, K.; Lobban, C.S. Seaweed Ecology and Physiology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014; pp. 1–551.
- Clay, J. Freeze the footprint of food. Nature 2011, 475, 287–289.
This entry is offline, you can click here to edit this entry!
