Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Neurosciences
Neurological and psychiatric patients have increased dramatically in number in the past few decades. Effective treatments for these diseases and disorders are limited due to heterogeneous and unclear pathogenic mechanisms.
- enteroendocrine cells
- enterochromaffin
- GLP1
- GLP2
1. Introduction
The number of patients suffering from neurological and psychiatric disorders has increased dramatically in the past few decades. According to the World Health Organization (WHO) epidemiology statistics, the number of Parkinson’s disease (PD) patients has doubled within the last 25 years [1]. Moreover, recent updates from the WHO indicate that there are nearly a billion people suffering from mental disorders, while approximately 280 million people suffer from depression around the world [2,3]. However, effective treatments for neurological and psychiatric disorders are currently limited due to the heterogeneous disease pathogenesis and targets of treatments. For example, it has been shown that visceral pain or depression patients sometimes express resistance to treatment [4,5,6,7]. Therefore, in order to develop novel therapeutic strategies, the exploration of novel aspects of neurological or psychiatric pathogenic mechanisms is urgently required.
Enteroendocrine cells (EECs) are chemosensory cells residing in the intestinal epithelium, and they function as important sensors monitoring changes in the lumen of the gastrointestinal (GI) tract. The EECs orchestrate not only the communication with luminal microorganisms via microbial metabolites, but also the communication with host body systems via neuroendocrine hormones (Figure 1 and Figure 2). For example, epithelial EECs continuously respond to short-chain fatty acids (SCFAs) generated by luminal microorganisms via free fatty acid receptor 2 and 3 (FFAR2/3). Following this transceptor or receptor activation, EECs secrete pre-made peptide hormones to conduct paracrine and endocrine functions [8,9,10,11]. Further, the neuropod structure of EECs allows direct or indirect signal transductions to enteric glia cells and enteric neurons [12,13]. An increasing body of evidence indicates the unique involvement or pathogenic role of the gut–brain axis and gut microbiota in neurological and psychiatric disorders, in which EECs might participate [14,15,16,17,18,19].
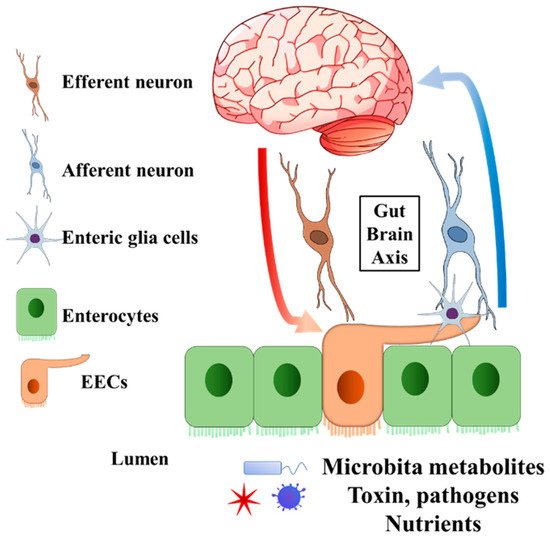
Figure 1. General structure of EECs involved in gut–brain axis. The EECs present receptors on the brush border to sense the microbiota metabolites, toxins, pathogens, and nutrients in the lumen. Enteric glia cells and neurons connect to EECs. The secreted endocrine molecules affect afferent neuron signalling directly and (or) indirectly via EECs enteric glia cells. Efferent neurons bring the signal into the central nervous system. On the other hand, the central nervous system can pass the signal to EECs through efferent neurons. EECs, enteroendocrine cells.
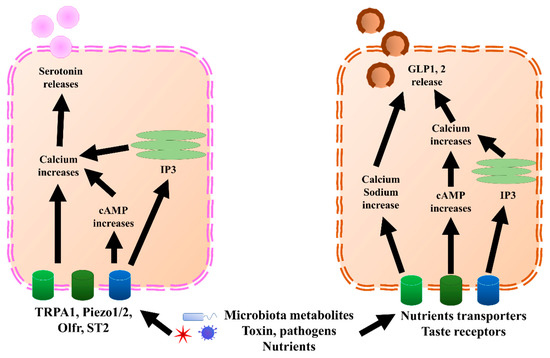
Figure 2. GLP1, 2, and serotonin secretion mechanisms in EECs and ECs, respectively. In ECs, TRPA1, piezol 1/2, Olfr, and ST2 bind to microbiota metabolites, pathogens, or nutrients, which increases calcium levels through directly increasing cAMP or endoplasmic reticulum IP3. Once the calcium level increases, it triggers the release of serotonin. Similar to ECs, EECs sense microbiota metabolites, toxins, pathogens, and nutrients through nutrient transporters and taste receptors. Further, they trigger an increase in calcium and sodium by directly increasing cAMP or endoplasmic reticulum IP3. Once the calcium level increases, it triggers the release of GLP1 and GLP2. cAMP, cyclic adenosine monophosphate; ECs, enterochromaffin cells; EECs, enteroendocrine cells; GLP1, glucagon-like peptide 1; GLP2, glucagon-like peptide 2; IP3, inositol trisphosphate; Olfr, olfactory receptor; TRPA1, transient receptor potential ankyrin 1.
2. Enteroendocrine Cell Functions That Might Be Related to Neurological and Psychiatric Disorders
EECs are located in the epithelium throughout the GI tract. They dynamically produce and store various peptide hormones and bioactive components, depending on the intestinal segments and epithelial homeostasis status. The regulation of EECs’ content profile, as well as their functions in energy metabolism and roles as incretins, has been reviewed elsewhere [20,21]. EECs can be further categorized into multiple subtypes, depending on their endocrine molecules production and secretion. For example, G cells can be identified by the secretion of gastrin; K cells uniquely secrete gastric inhibitory peptides; L cells produce and secrete GLP1, GLP2, PYY, and oxyntomodulin; I cells produce cholecystokinin (CCK); N cells secrete neurotensin; S cells secrete secretin; enterochromaffin cells (ECs) secrete serotonin; and enterochromaffin-like cells secrete histamine [20,21]. Here, we only focus on the subtypes of EECs that are potentially involved in neurological and psychiatric disorders.
L cells mostly secrete GLP1, GLP2, PYY, and oxyntomodulin, although PYY might also be co-expressed with gastrin, which is mostly secreted by G cells [22]. GLP1, GLP2, and PYY, which are secreted by L cells, and serotonin, secreted predominantly by ECs (Figure 2), are discussed in this paper. GLP1 and GLP2 have been shown to correlate with multiple neurological disorders. Serotonin also showed a correlation with depression and visceral pain, although this is still under debate. Besides incretin functions, GLP1 has been shown to exert anti-inflammatory effects in both the GI tract and central nervous system (CNS) [23,24,25]. Moreover, GLP1 possesses neuroprotective effects and triggers neurogenesis [26,27,28,29,30,31]. New evidence suggested that GLP1 and glucagon-like peptide 1 receptor (GLP1R), a receptor of GLP1, have protective effects on hypothalamic inflammation and leptin sensitivity in mice [25,32]. Despite the well-known source and their effects within the CNS, the GLP1 derived from intestinal EECs has also been suggested to play a role in neurological pathology, due to the feature wherein GLP1 is able to pass through the brain–blood barrier [33,34,35]. Similar to GLP1, L-cell-secreted GLP2 also possesses anti-inflammation effects [36,37,38]. In cows, GLP2 administration increased the intestinal villi height, mucosal surface, and proliferating cells, and decreased inflammation [39]. Further, GLP2 has a neuroprotective effect and can trigger neurogenesis in a similar manner as GLP1 [29,37,40,41,42]. Interestingly, the anti-inflammatory effects of other components of EEC content have recently been revealed, including PYY [43,44] and serotonin [45,46], which are likely associated with neuroinflammation. Therefore, accumulating evidence suggests that EECs and ECs could play important pathogenic and regulatory roles in neurological and psychiatric disorders.
3. Enteroendocrine Cells in Parkinson’s Disease
PD is a common movement disorder that was originally characterized as a neurodegenerative disorder due to the loss of dopaminergic neurons and accumulated aggregation of α-synuclein fibrils (called Lewy bodies) (reviewed elsewhere previously [47]). However, studies have shown a new pathogenic aspect of PD, which could be linked to intestinal disorders, as well as to changes in intestinal microbiota and metabolites [15,48,49]. For instance, inflammatory bowel disease (IBD) has increased by 22 to 35% regarding the incidence of PD [50]. In addition, Sampson et al. reported that the GI microbiota was required for motor deficits, microglia activation, and α-synuclein pathology (PD symptoms), in a germ-free mice model overexpressing α-synuclein. Further, their results indicated that the microbial metabolites produced in PD patients enhanced the pathophysiology of PD [15]. Although with a negative correlation, others also found an association among the GI microbiota, the total faecal SCFAs, and PD incidence [48]. Researchers hypothesized that the origin of PD might lie in the enteric nervous system (ENS) [51,52]. Accordingly, α-synuclein was detected in GI mucosa in early PD patients [53,54].
Given the important luminal chemo-sensing and neuroendocrine functions of EECs, these recent results point to a hypothesis that EECs contribute to and regulate the pathogenesis of PD. Interestingly, in human intestinal tissue, the α-synuclein that triggers PD was colocalized with EECs, such as L cells and K cells [55,56]. Although the authors have not confirmed the original secretion location of the α-synuclein, the data in these studies strengthen the possibility of EECs’ involvement in PD progression.
Two potential mechanisms of EECs’ contribution in PD pathogenesis have been proposed. On one hand, the EECs are likely to be a source of α-synuclein, which is generated in response to specific microbial activation. Thereafter, the α-synuclein is transported into the brain via nerves, leading to the accumulation of α-synuclein [57] (Figure 3a). In line with this hypothesis, a very recent research work revealed the potential mechanisms. The authors identified an increased population of microorganism Akkermansia muciniphila in the guts of PD patients. The metabolites of this microorganism initiated α-synuclein aggregation in EECs, via activation of ryanodine receptor (RyR), calcium ion (Ca2+) release, and increased mitochondrial reactive oxygen species (ROS) generation [58] (Figure 3b). Moreover, a newly published paper indicated that another microbial metabolite, sodium butyrate, increased the α-synuclein mRNA expression in EECs through the autophagy-related 5 (Atg5) dependent autophagy pathway [59]. Holmqvist et al. provided evidence that α-synuclein was able to move from the intestine to the brain in rats [60]. Further, the transportation of α-synuclein from EECs to neurons requires GTPase called Ras-related protein Rab-35 (Rab35) and cell-to-cell contact, which is in line with the EECs’ characteristics [61].
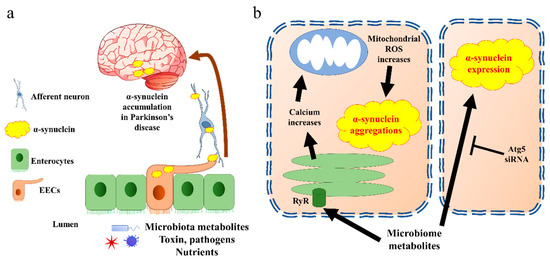
Figure 3. α-synuclein accumulates in Parkinson’s disease through EECs. (a) The general pathway by which EECs trigger α-synuclein transfer into brain. Aggregated α-synuclein produced by EECs is transported into brain through afferent neurons and vagal nerve. (b) Cell signalling of α-synuclein aggregation present in EECs. The EECs present receptors on the brush border to sense the microbiota metabolites. Triggering of endoplasmic reticulum releases calcium through RyR. The increase in calcium induces reactive oxygen species (ROS) synthesis in mitochondria, which further creates α-synuclein aggregates. Microbiota metabolites also increase α-synuclein expression through Atg5 pathway in EECs. Atg5, autophagy-related 5; EECs, enteroendocrine cells; ROS, reactive oxygen species; RyR, ryanodine receptor.
On the other hand, the EECs’ secretion could also be suppressed by alterations in luminal SCFA concentrations and profiles. This could be the consequence of changes in specific microbes, which then increase the systemic inflammation, and this eventually enhances the progression of PD [62,63,64]. It was suggested that sodium butyrate increased the pro-inflammatory cytokines and α-synuclein mRNA expression in an EECs cell line and neuroblast cell line treated with EECs conditional medium [59]. Further, the EECs facilitate α-synuclein transport, which could trigger inflammation responses in microglia [65,66]. In contrast, a study in a PD mouse model suggested that the oral administration of butyrate could have protective effects on the neurobehavioral impairment via increased EEC activities, such as increased colonic GLP1 expression and brain GLP1R gene expression [67]. A recent animal study also indicated the neuroprotective effect of GLP1, triggered by chlorogenic acid [31]. These conflicting characteristics of EECs might be due to the variations in EECs’ homeostasis status or the hormone composition of EECs. In other words, the EECs that secrete GLP1 could be beneficial in terms of inflammation reduction, while the EECs that cannot secrete GLP1 but produce α-synuclein could be harmful. However, the detailed mechanism for either hypothesis is still unclear, especially regarding the extent to which EECs contribute to inflammation in PD patients. Future study will be needed to investigate the detailed mechanisms of EECs in PD progression.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10102577
This entry is offline, you can click here to edit this entry!
