Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Due to the influence of various stressful stimuli, psychological stress alters the homeostasis of the organism. Consequently, the organism reacts, and the sympathetic-adrenal-medullary (SAM) system and the hypothalamic-pituitary-adrenal (HPA) axis are activated, producing and releasing specific hormones. In addition to acute stress, chronic psychological stress also activates the HPA axis, which causes elevated glucocorticoid levels.
- students
- saliva
- biomarkers
- cortisol
- amylase
- HPA axis
1. Introduction
Due to the influence of various stressful stimuli, psychological stress alters the homeostasis of the organism. Consequently, the organism reacts, and the sympathetic-adrenal-medullary (SAM) system and the hypothalamic-pituitary-adrenal (HPA) axis are activated, producing and releasing specific hormones [1][2]. Therefore, the psychological response to emotional stress also modulates the functions of the immune system, the autonomic nervous system (ANS), levels of hypothalamic and pituitary hormones, neuropeptides, cytokines, and other factors involved in this network [3][4].
The stress response results in changes at the molecular level of the whole body (Figure 1) [5][6][7][8][9]. Thus, short-term and long-term effects of stress are associated with changes/alterations in HPA axis functioning, which alters glucocorticoid levels and may influence different health outcomes [10]. The stress response includes the connection between the central nervous system (CNS) and the immune system, with bidirectional connections. In addition, adaptation to stress is a very important mechanism in the body’s response to stress. Thus, the effectiveness of the reaction to stress depends on the type, intensity, and duration of stress as well as the characteristics of the person [11].
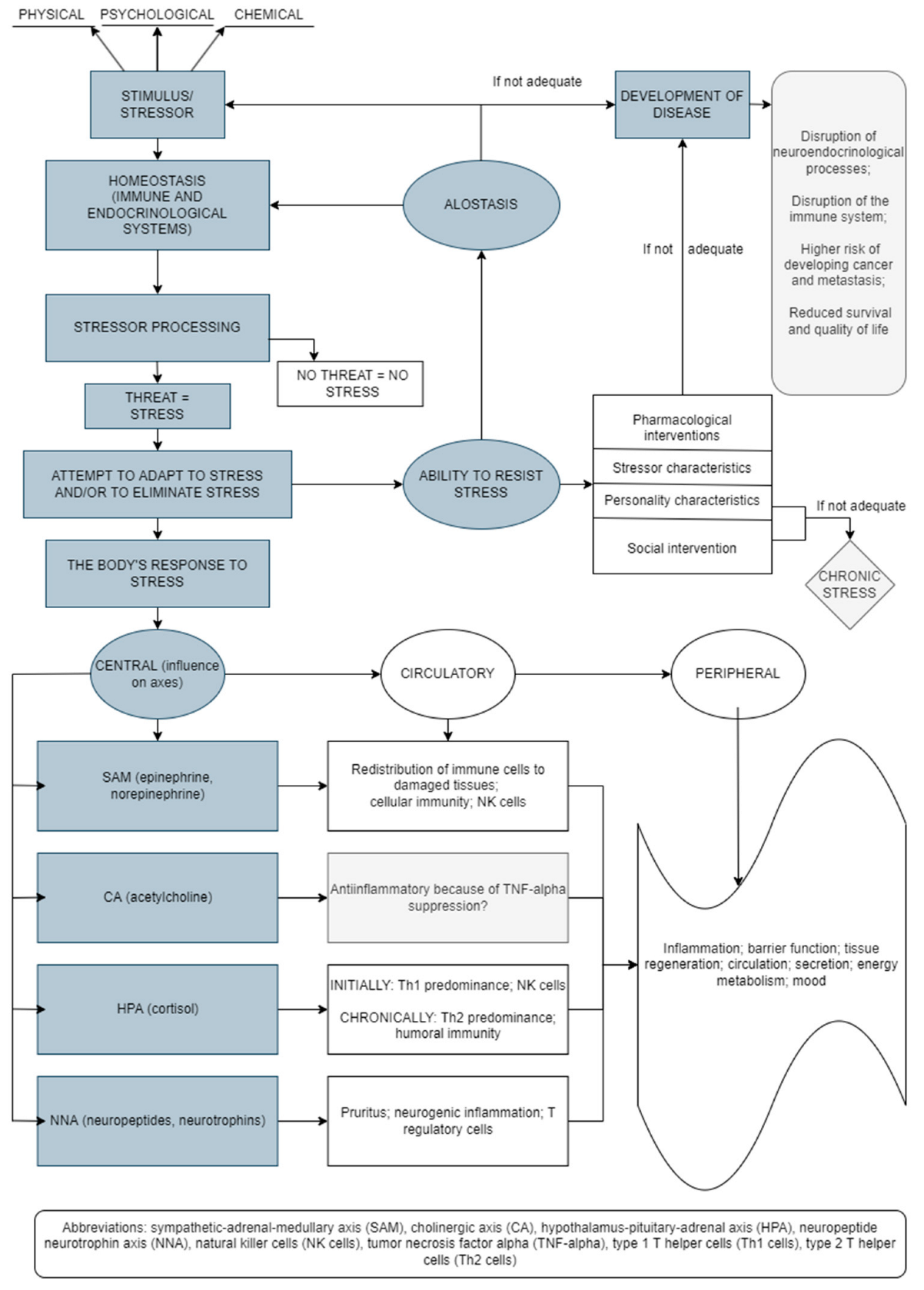
Figure 1. Stress-induced bodily reactions and factors involved in this network (an original scheme based on current literature data).
2. Physiology of Acute and Chronic Stress Reactions and the HPA Axis
A stress reaction implies greater activity of the HPA axis. The organism’s response to acute stress involves a complex process/network which is mediated by the HPA axis and involves changes in psychological and social factors. In addition to acute stress, chronic psychological stress also activates the HPA axis, which causes elevated glucocorticoid levels [12]. It is well-known that this release of stress-associated glucocorticoids can impair memory and cognitive functions [1][13][14][15]. Chronic stress can also weaken the immune response (as confirmed by the determination of antibody responses to vaccines) and can cause or contribute to different diseases such as cardiovascular, endocrine, gastrointestinal diseases, and others [12]. The research literature indicates that acute stress can also change levels of different immune factors, but by increasing them [16]. Increased cortisol levels lead to hyperactivity of the HPA axis, while disorders of the HPA axis present an increased risk for developing various diseases and conditions [17][18]. However, research on the impact of naturally stressful events on diseases has given contradictory results about the increased risk of developing certain diseases [10].
The peripheral and central nervous systems are important factors in a complex network of reactions involved in the body’s response to stress [11]. The brain receives and processes various neurosensory impulses (cortical, limbic, visual, somatosensory, nociceptive, visceral, etc.), including signals coming from the blood (hormones, cytokines, mediators). During psychological stress, the crucial role belongs to the sympathetic nervous system, mostly to the ANS, which controls the functions of the internal organs via the sympathetic and parasympathetic nervous systems [11]. The acute stress response includes, firstly, the registration of the stressful stimulus, which is transmitted from the cerebral cortex to the nucleus of the brainstem locus coeruleus/norepinephrine (LC/NA) and pons, whose neurons stimulate (via receptors) the release of catecholamines from the adrenal medulla directly into circulation. This release of catecholamines affects the body by making an individual more alert and cautious, activating defensive behavior patterns, usually with higher aggression, stimulating the cardiovascular and respiratory systems and inhibiting the gastrointestinal system. Consequently, the increase in catecholamine levels also results in increased levels of plasma glucose and fatty acids. These reactions are crucial for survival and protection of the organism during stressful periods [11].
Stressors stimulate the hypothalamus to secrete corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP), which leads to the release of adrenocorticotropic hormone (ACTH) from the anterior pituitary lobe. This is followed by activation of adrenal gland cells, which produce and release glucocorticoids. Additionally, HPA axis activation is crucial for the body’s catabolic processes and for supplying the organism with energy. Increased plasma cortisol concentration stimulates the liver to perform the process of gluconeogenesis and causes insulin resistance in peripheral tissues [19]. Consequently, glucocorticoids are crucial in controlling the duration of the stress response. Activation of the stress system causes clinical manifestations that include physiological reactions (oxygen and nutrients are directed to the CNS and other body systems) and behavioral reactions (e.g., increased excitement, vigilance, caution, focus, euphoria, or dysphoria, etc.) [11]. Clearly, multiple systems are involved in the adaptation of the body to new circumstances, and numerous processes (e.g., lipolysis and gluconeogenesis) take place to supply the body with energy at such times. At the same time, the activities of other systems (gastrointestinal, reproductive, and immune systems) are inhibited [11].
Successful adaptation to stress activates the body’s mechanisms that control and inhibit stress reactions—in other words, they prevent an excessive response to stress. Without these mechanisms in place, the stress response would be too intense and prolonged, going beyond successful adjustment, thus potentially causing a pathological condition. There is a constant, dynamic balance between the stimulatory and inhibitory mechanisms of a stress reaction, but the mechanisms that inhibit stress reactions prevail during the recovery process. A proper response to stress involves timely calming or neutralization of stress effects. Catabolic processes predominate after a stressful situation, i.e., energy mobilization occurs due to the effects of stress hormones (catecholamines, cortisol, glucagon) [20]. During rest and recovery, anabolic processes predominate due to the influence of growth hormone and gonadal steroids important for healing and growth. Thus, to establish the homeostasis that each cell tries to achieve, this balance between the anabolic and catabolic processes is needed [11].
Psychological stress is associated with various diseases and conditions, including cardiovascular diseases (e.g., hypertension), diabetes, gastrointestinal disorders, increased susceptibility to infections, autoimmune disorders, malignant diseases, and others [21]. In addition, high levels of stress have an impact on mental health and can lead to drug abuse, reduced work efficiency, absenteeism, and other behaviors associated with poor mental or overall health [2][22][23]. Literature data illustrates how medical interventions (pharmacological, psychotherapeutic) try to reduce the impact of cortisol in response to stressful stimuli [10].
It is important to mention, here, that gender influences the stress response—psychoendocrinological studies have reported lower stress responses in women than in men [24][25][26]. In addition, a subjectively higher perception of stress in women, and their typical cognitive styles, likely contribute to their higher risk of developing psychological disorders and anxiety disorders [27]. Gender’s significant impact on the immune system has been confirmed by several studies that analyzed the acute stress response. Notably, the menstrual cycle affects endocrine and immune variations by altering the level of circulating cytokines or growth factors [28]. A blunting impact on the stress response may be caused by fluctuations of gonadal steroids during the menstrual cycle and may also be related to oral contraceptive use [27][29]. Thus, Helbig et al. [27] showed a stronger cortisol response in women in the luteal phase than in those in the follicular phase or in those taking oral contraceptives. Additionally, salivary secretion differs between the sexes and may be associated with variations in the secretion of gonadal steroids and ANS regulation of salivary glands [30].
This entry is adapted from the peer-reviewed paper 10.3390/bs12100400
References
- Ouda, S.; Alaki, S.; Safi, M.A.; Nadhreen, A.; Johani, K.A. Salivary Stress Biomarkers–Are They predictors of Academic Assessment Exams Stress. J. Clin. Exp. Pathol. 2016, 15, 276–279.
- O’Connor, D.B.; Thayer, J.F.; Vedhara, K. Stress and Health: A Review of Psychobiological Processes. Annu. Rev. Psychol. 2020, 72, 663–688.
- Ravishankar, T.L.; Ain, T.S.; Gowhar, O. Effect of Academic Stress on Plaque and Gingival Health among Dental Students of Moradabad, India. J. Int. Acad. Periodontol. 2014, 16, 115–120.
- Dhama, K.; Latheef, S.K.; Dadar, M.; Samad, H.A.; Munjal, A.; Khandia, R.; Karthik, K.; Tiwari, R.; Yatoo, M.I.; Bhatt, P.; et al. Biomarkers in Stress Related Diseases/Disorders: Diagnostic, Prognostic, and Therapeutic Values. Front. Mol. Biosci. 2019, 6, 91.
- Ader, R.; Cohen, N.; Felten, D. Psychoneuroimmunology: Interactions between the Nervous System and the Immune System. Lancet 1995, 345, 99–103.
- McEwen, B.S. Protective and Damaging Effects of Stress Mediators. N. Engl. J. Med. 1998, 338, 171–179.
- McEwen, B.S. Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiol. Rev. 2007, 87, 873–904.
- Besedovsky, H.O.; Rey, A.D. Physiology of Psychoneuroimmunology: A Personal View. Brain Behav. Immun. 2007, 21, 34–44.
- Lugović-Mihić, L.; Cvitanović, H.; Djaković, I.; Kuna, M.; Šešerko, A. The Influence of Psychological Stress on HPV Infection Manifestations and Carcinogenesis. Cell Physiol. Biochem. 2021, 55, 71–88.
- Gaab, J.; Sonderegger, L.; Scherrer, S.; Ehlert, U. Psychoneuroendocrine Effects of Cognitive-Behavioral Stress Management in a Naturalistic Setting—A Randomized Controlled Trial. Psychoneuroendocrinology 2006, 31, 428–438.
- Ivković, N.; Božović, Đ.; Račić, M.; Popović-Grubač, D.; Davidović, B. Biomarkers of Stress in Saliva. Acta Fac. Med. Naissensis 2015, 32, 91–99.
- McGregor, B.A.; Murphy, K.M.; Albano, D.L.; Ceballos, R.M. Stress, Cortisol, and B Lymphocytes: A Novel Approach to Understanding Academic Stress and Immune Function. Stress 2016, 19, 185–191.
- Ng, V.; Koh, D.; Mok, B.Y.Y.; Chia, S.-E.; Lim, L.-P. Salivary Biomarkers Associated with Academic Assessment Stress among Dental Undergraduates. J. Dent. Educ. 2003, 67, 1091–1094.
- de Quervain, D.; Schwabe, L.; Roozendaal, B. Stress, Glucocorticoids and Memory: Implications for Treating Fear-Related Disorders. Nat. Rev. Neurosci. 2016, 18, 7–19.
- Langer, K.; Wolf, O.T.; Jentsch, V.L. Delayed Effects of Acute Stress on Cognitive Emotion Regulation. Psychoneuroendocrinology 2021, 125, 105101.
- Benham, G.; Nash, M.R.; Baldwin, D.R. A Comparison of Changes in Secretory Immunoglobulin a Following a Stress-Inducing and Stress-Reducing Task. Stress Health 2009, 25, 81–90.
- Lenaert, B.; Barry, T.J.; Schruers, K.; Vervliet, B.; Hermans, D. Emotional Attentional Control Predicts Changes in Diurnal Cortisol Secretion Following Exposure to a Prolonged Psychosocial Stressor. Psychoneuroendocrinology 2016, 63, 291–295.
- Leistner, C.; Menke, A. Hypothalamic-Pituitary-Adrenal Axis and Stress. Chapter 4: Sex Differences in Neurology and Psychiatry. In Handbook of Clinical Neurology; Lanzenberger, R.G.S., Kranz, G.S., Savic, I., Eds.; Elsevier, B.V.: Amsterdam, The Netherlands, 2020; Volume 175, pp. 55–64.
- Lelou, E.; Corlu, A.; Nesseler, N.; Rauch, C.; Mallédant, Y.; Seguin, P.; Aninat, C. The Role of Catecholamines in Pathophysiological Liver Processes. Cells 2022, 11, 1021.
- Jones, B.J.; Tan, T.; Bloom, S.R. Minireview: Glucagon in Stress and Energy Homeostasis. Endocrinology 2012, 153, 1049–1054.
- Knowles, S.R.; Nelson, E.A.; Palombo, E.A. Investigating the Role of Perceived Stress on Bacterial Flora Activity and Salivary Cortisol Secretion: A Possible Mechanism Underlying Susceptibility to Illness. Biol. Psychol. 2008, 77, 132–137.
- McKerrow, I.; Carney, P.A.; Caretta-Weyer, H.; Furnari, M.; Miller Juve, A. Trends in Medical Students’ Stress, Physical, and Emotional Health throughout Training. Med. Educ. Online 2020, 25, 1709278.
- Lever-van Milligen, B.A.; Lamers, F.; Smit, J.H.; Penninx, B.W.J.H. Physiological Stress Markers, Mental Health and Objective Physical Function. J. Psychosom. Res. 2020, 133, 109996.
- Deinzer, R.; Granrath, N.; Stuhl, H.; Twork, L.; Idel, H.; Waschul, B.; Herforth, A. Acute Stress Effects on Local IL-1β Responses to Pathogens in a Human in Vivo Model. Brain Behav. Immun. 2004, 18, 458–467.
- Hamidovic, A.; Van Hedger, K.; Choi, S.H.; Flowers, S.; Wardle, M.; Childs, E. Quantitative Meta-Analysis of Heart Rate Variability Finds Reduced Parasympathetic Cardiac Tone in Women Compared to Men during Laboratory-Based Social Stress. Neurosci. Biobehav. Rev. 2020, 114, 194–200.
- Sze, Y.; Brunton, P.J. Sex, Stress and Steroids. Eur. J. Neurosci. 2019, 52, 2487–2515.
- Helbig, S.; Backhaus, J. Sex Differences in a Real Academic Stressor, Cognitive Appraisal and the Cortisol Response. Physiol. Behav. 2017, 179, 67–74.
- Kamezaki, Y.; Katsuura, S.; Kuwano, Y.; Tanahashi, T.; Rokutan, K. Circulating Cytokine Signatures in Healthy Medical Students Exposed to Academic Examination Stress. Psychophysiology 2012, 49, 991–997.
- Schoofs, D.; Hartmann, R.; Wolf, O.T. Neuroendocrine Stress Responses to an Oral Academic Examination: No Strong Influence of Sex, Repeated Participation and Personality Traits. Stress 2008, 11, 52–61.
- Sangiorgio, J.P.M.; Seixas, G.F.; De Paula Ramos, S.; Dezan-Garbelini, C.C. Salivary Levels of SIgA and Perceived Stress among Dental Students. J. Health Biol. Sci. 2017, 6, 9.
This entry is offline, you can click here to edit this entry!
