Minimally invasive mitral valve surgery is evolving rapidly since the early 1990’s and is now increasingly adopted as the standard approach for mitral valve surgery. It has a long and challenging learning curve and there are many considerations regarding technique, planning and patient selection when starting a minimally invasive program.
- minimally invasive mitral valve surgery
- learning curve
- decision making
[1][2][3][4][5][6][7][8][9][10][11][12]1. Introduction
2. Learning Curve of Minimally Invasive Mitral Valve Surgery
3. Benefits and Limitations of Minimally Invasive Access
3.1. Benefits of Minimally Invasive Access
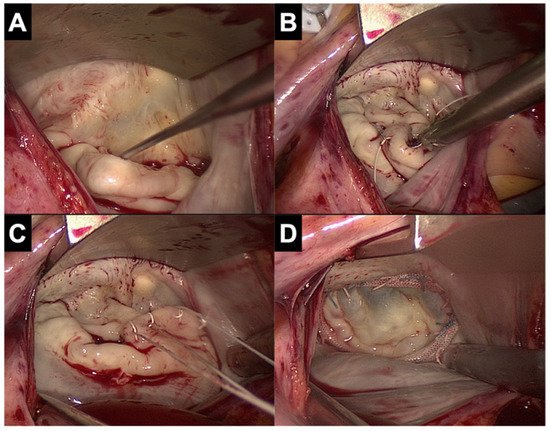
3.2. Limitations of Minimally Invasive Access
4. Considerations in Pre-Operative Planning and Patient Selection during the Learning Curve
4.1. Pre-Operative Planning and Imaging
4.2. Patient Selection
| Significant Aortic, Iliac, or Femoral Disease That Prevents Safe Retrograde Arterial Perfusion |
|---|
| Left ventricular ejection fraction < 25% |
| Severe right ventricular dysfunction |
| Pulmonary artery pressure > 70 mmHg |
| Aorta > 4 cm if endo-aortic balloon being used |
| Significant mitral annular calcification |
| Patients with more than mild aortic regurgitation |
| Kyphoscoliosis and pectus excavatum |
| Morbidly obese and extremely muscular patients |
| Previous right thoracotomy or expected adhesions in the right chest |
| Advanced renal- or liver disease, significant pulmonary disease |
4.3. Considerations of Mitral Valve Pathology during Learning Curve of MIMVS
5. Available techniques and safety considerations
5.1. Safety of the minimally invasive techniques
Multiple techniques are available at each step of surgery (e.g. chest access, cardiopulmonary bypass configuration, aortic occlusion). In the early days of MIMVS, there were concerns about the safety of this technique, especially with regard to aortic injury and stroke (23). Since the 1990s, results have improved significantly due to better pre-operative planning, adjustments to surgical technique and as more experienced centers have become familiar with the technique. A recent STS database study from 2021 and recent meta-analysis show similar results for MIMVS versus sternotomy (4-9, 11, 39, 40). To initiate the MIMVS program, an institute should begin with the lowest risk patients with non-complex valve pathology and the most appropriate anatomy for the access. In a more advanced stage, more advanced techniques can be applied to expand the appropriate patient population that will benefit from the minimally invasive technique. However, moving towards more complex and less invasive techniques should never compromise operative safety and the valve repairability. Available techniques for each step of the operation (access, cardiopulmonary bypass, aortic occlusion) are discussed in the section below.
5.2. Less invasive surgical access and visualization of the mitral valve
In literature, access for MIMVS has been described through lower mini sternotomy, right parasternal incision or right anterolateral mini-thoracotomy. Different techniques have been described for the right mini-thoracotomy (surgery under direct vision with video assistance, or totally thoracoscopic approach and robotic assisted approach) and it is important that the team can adapt to one technique (41). Highly specialized centers use a peri areolar access for improved aesthetic results (42, 43), but the most common incision site is the right mini thoracotomy. For the direct view technique, the thoracotomy incision is placed slightly more lateral compared to the totally thoracoscopic technique, in which the incision is placed more anterior and the camera is placed more laterally, directly in line with the mitral valve. Both 2D and 3D cameras can be used, of which the 3D camera provides a very realistic image of the intrathoracic space, allowing best estimation of the depth for optimal accuracy. All instruments used are specialized long shafted instruments for endoscopic cardiac surgery.
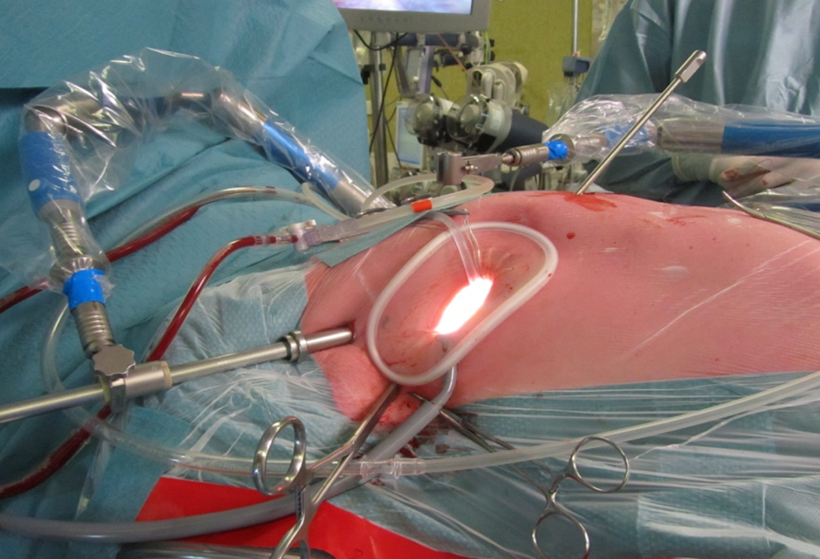
Figure 3. Setup of a MIMVS procedure by right anterolateral mini thoracotomy. Starting from the bottom of the soft tissue retractor in clockwise direction: Carbon dioxide insufflator, Transthoracic Chitwood clamp, video port and left atrial retractor.
The robotic assisted approach is the most complex to learn and has a learning curve beyond mini thoracotomy (44). In the robotic assisted technique, a 3D camera is used to visualize the chest and robotic arms are installed through ports and a variety of instruments can be inserted (e.g. scissors, diathermia, forceps). Lack of tactile feedback has not been experienced as troublesome in experienced robotic surgeons due to the realistic image with the 3D camera and the intuitive instruments with 360-degree freedom of motion. The disadvantages of this technique are more pain experience at the thoracic incisions (ports), higher costs and complex planning due to the availability of the robot (45).
5.3. Cardiopulmonary bypass: arterial cannulation
Cardiopulmonary bypass can be organized peripherally and centrally with a variety of configurations for many reasons. For peripheral cannulation, arterial access is most frequently obtained through the femoral artery, percutaneously (Seldinger technique) or via a groin incision, as it is a simple and reproducible technique that does not interfere with the surgical field. Whenever the femoral artery is inaccessible (e.g. in case of severe ilio-femoral calcifications, stenosis and tortuosity or thrombus in the thoraco-abdominal aorta), an 8mm Dacron graft can be sewn on the subclavian artery for arterial cannulation (46).
For central arterial cannulation, the cannula enters the chest through the mini thoracotomy or through an extra port and is inserted directly into the aorta, comparable to traditional open cardiac surgery. The advantage of direct aortic or subclavian artery cannulation is an antegrade blood flow towards the carotid arteries which decreases the risk of stroke in patients with atherosclerotic disease or intra-aortic thrombus, in whom plaques and thrombi can be embolized during retrograde perfusion, causing stroke. However, an extra cannula in the surgical working field can interfere the surgery. A recent systematic review showed that retrograde perfusion might increase stroke risk in MIMVS compared to antegrade perfusion, but has no influence on mortality and renal failure (47). However, as mentioned by the authors, the included studies were retrospective observational studies without a pre-operative imaging protocol and therefore the association of stroke with retrograde perfusion might be biased by selection. There was only one study with a pre-operative imaging protocol, in which patients with tortuous and/or atheromatous aortoiliac-femoral arteries were selected for central cannulation. There was no difference in stroke rate of both cannulation techniques, suggesting that these patients could benefit from a modified cannulation strategy (48). In the early experience of a center, peripheral femoral cannulation is the safest, simplest and most frequently applied technique. The pre-operative CT scan will aid in selection of patients without the complicating vascular anatomy to reduce the risk of stroke and vascular complications. In a more advanced stage, a modified cannulation strategy will expand the patient population that benefit from the minimally invasive access.
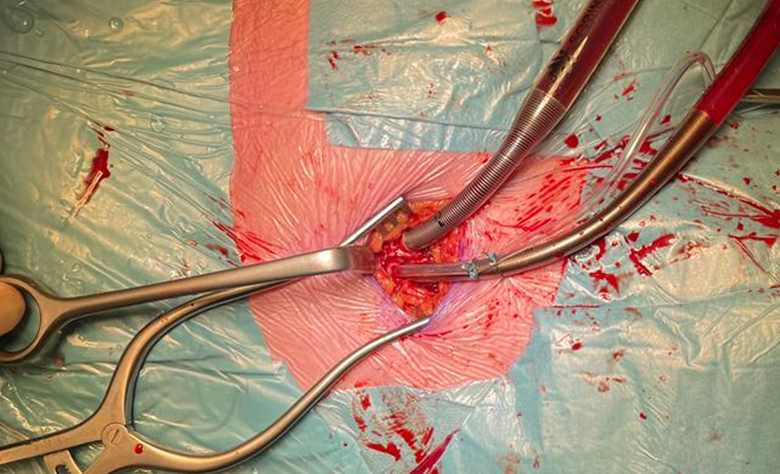
Figure 4. Peripheral arterial and venous cannulation in the right groin. Standard arterial cannula for direct aortic cross clamp technique. When using an endoaortic balloon, there is a sidearm in the arterial cannula to insert the balloon.
5.4. Cardiopulmonary bypass: Venous cannulation
As in arterial cannulation, peripheral venous cannulation is most frequently used in MIMVS. Access is obtained through the femoral vein, percutaneously (Seldinger technique) or through a groin incision, by inserting a two staged cannula positioned with the tip in the superior vena cava under TEE guidance, draining the superior and inferior caval vein. For tricuspid valve surgery or closure of an atrial septum defect, it is preferable to drain both caval veins separately, snaring both caval veins when opening the right atrium to prevent air lock in the extracorporeal circulation. When preferred an additional cannula can be inserted through the interna jugular or subclavian vein next to the femoral cannula (with the tip just below the diafragm) for separate caval venous drainage. This technique is also indicated in mitral valve surgery in patients with a vena cava filter (40) to prevent dislocation of the filter. Central venous cannulation is less frequently applied in MIMVS since it is obstructing the surgical field. It is recommended to familiarize the team with the above techniques before starting a MIMVS program. In the early experience, inserting a two staged venous cannula in the femoral vein is the simplest to learn. However, pre-operative CT planning to discover anatomical variants (e.g. Interrupted inferior vena cava or persistent left vena cava superior) and strict guidance with TEE during the insertion of the cannula is mandatory to avoid displacement injuries and to ensure adequate drainage.
5.5. Techniques for aortic occlusion and de-airing
In literature, there are two techniques described for aortic cross clamping in MIMVS: the endo-aortic balloon occlusion and the direct transthoracic aortic clamp (e.g. Chitwood clamp).Compared to conventional heart surgery, the surgeon is most accustomed to the direct transthoracic aortic cross-clamp and is therefore most easy to handle (49). For the administration of cardioplegia in this technique, an aortic root vent must be placed. Removal of this vent after releasing the clamp can be challenging. One of the techniques is to remove the aortic root vent after the de-airing process and before releasing the clamp. An alternative technique is to administer retrograde cardioplegia in the coronary sinus. A catheter can be placed either by the anesthetist or the surgeon under direct view after snaring both the superior and inferior caval vein. In this technique, the patient is placed in Trendelenburg during the whole procedure and a left vent can be inserted for de-airing. Using this aorta clamp, the surgeon has to take care not to damage the left atrial appendage or the pulmonary artery. An alternative for direct aortic occlusion with a clamp is the endo-aortic balloon, which is inserted via a side branch of the arterial femoral cannula. The use of the endo balloon was recently described in detail by van Praet et al (50, 51). In brief, the balloon occludes the aorta and cardioplegia is administered through the balloon. During the aortic occlusion, close monitoring of the pressures in the aortic root, the balloon itself and the radial artery pressure are important. Migration of the balloon is possible whenever there are changes in the pressures, such as during the start and stop of administration of cardioplegia, opening the left atrium and changes in cardiopulmonary bypass flow. In redo-surgery, the balloon can be very useful to occlude the aorta without necessity to perform adhesiolysis.
After initial concerns of stroke and aortic injuries in the early days of MIMVS by the use of the endo-aortic balloon (52), these catastrophic complications have decreased (53). Knowledge and experience of regulating pressures in the aorta and balloon during the procedure have improved surgical outcome and studies have shown equal results compared to cross clamping (54, 55). Close communication with the perfusionist and anesthetist is necessary to prevent injuries associated with balloon migration such as stroke and aortic dissection.
When starting MIMVS, the direct transthoracic aortic clamp is the easiest to handle and for the team in training probably the safest technique. The advantage of the endo-aortic balloon is mostly in redo surgery as aortic occlusion can be obtained without adhesiolysis. However, such a technique should only be considered if the surgical team has sufficient experience in access and repair techniques to also focus on the endo-aortic balloon. Planning the procedure, it is important to choose for the technique which is the most appropriate for the surgical team experience.
5.6. Hypothermic fibrillary arrest
In selected patients, such as in redo surgery (especially in patients with patent internal mammary artery grafts after CABG), a calcified ascending aorta without safe clamping site (hostile aorta) or whenever the endo-aortic balloon is contra indicated, hypothermic fibrillary arrest can provide safe myocardial preservation. In patients with more than mild aortic valve regurgitation the vision can be hampered by retrograde blood. This can be overcome by introducing a second suction device in the left ventricle. In some patients a short stop of the heart lung machine at 25 degrees Celsius in Trendelenburg position can be very helpful to improve vision of especially the posterior annulus. De-airing can be done by leaving a vent in the left ventricle until sinus rhythm is restored and TEE confirm no residual air in the left ventricle. Experienced centers in MIMVS have reported outcomes of this technique with good results (7, 8, 10). However, since this technique requires extensive surgical experience, it should not be applied during the learning curve.
5.7. Valve repair and replacement techniques
Repair rates of MIMVS have been reported to be comparable to sternotomy approach (13, 14). All techniques for mitral valve repair are feasible through minimally invasive access, although there is a trend towards ‘respect’ rather than ‘resect’ in MIMVS. Recent studies have shown that the neochord technique showed better long-term results than the resection technique in patients with degenerative disease who underwent mitral valve repair through minimally invasive access (56, 57).
- Conversion to sternotomy
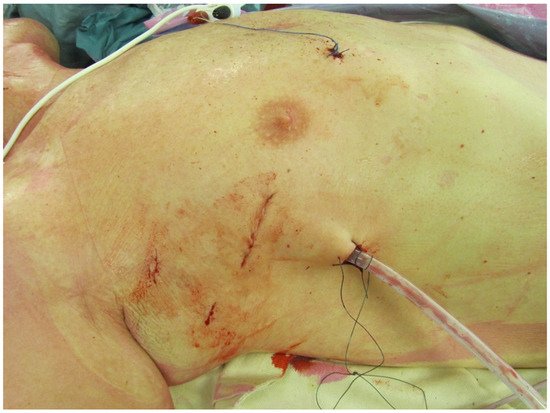
Conversion to sternotomy is reported at 1-2% in large series of experienced centers (4-6) and can be divided in an intended and unintended sternotomy. A perioperative unintended conversion is an emergency sternotomy due to a lesion of a major vascular structure or the heart (e.g. aortic dissection, atrioventricular dissociation). An intended conversion is a perioperative anticipated conversion to sternotomy due to inaccessible anatomy for MIMVS (e.g. unexpected peri-operative pulmonary adhesions). The Leipzig group have analyzed their conversions to sternotomy and reported main reasons were major bleeding (53%), severe pulmonary adhesions (18%) and type A aortic dissection (15%)(58). Potential bleeding site were the left atrial appendage (due to clamping) and left ventricular apex (due to the water tube for the water test). Awareness of these above-mentioned risks can prevent such complications. During the standard preparations on the operating room before minimally invasive surgery, marking of the incision for sternotomy can aid in such an emergency situation (figure 5).
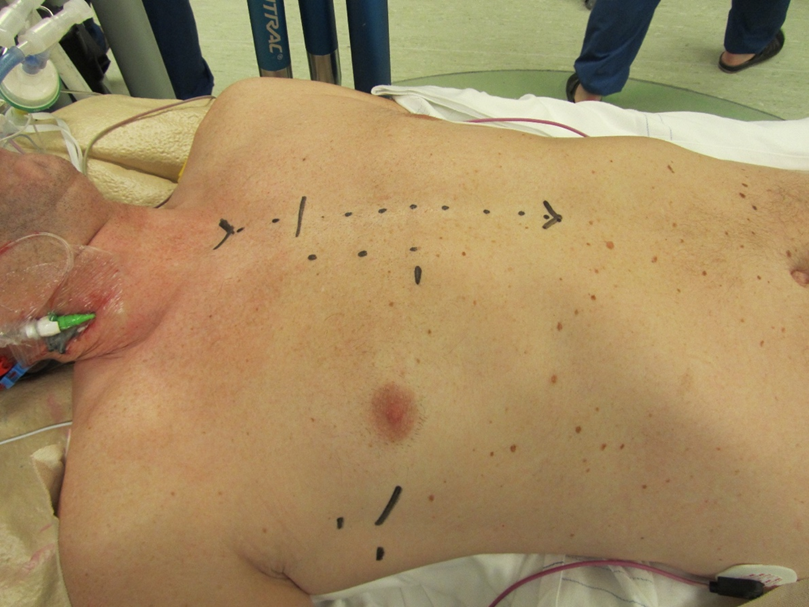
Figure 5. Marking of the incisions for MIMVS and the incision for conversion to sternotomy
In most cases of such an emergency conversion (such as for bleeding or aortic dissection), the patient is already on cardiopulmonary bypass. In case of a major bleeding, a cardiotomy suction sump can be placed in the chest, followed by an emergency sternotomy to repair whatever is causing the bleeding. Whenever there are pulmonary adhesions, there is enough time to perform a standard sternotomy to proceed with the surgery.
- Conclusion
MIMVS is increasingly adopted worldwide as the standard approach for mitral valve surgery due to less surgical trauma resulting in faster postoperative recovery and improved functional outcome. MIMVS is a complex technique with a long learning curve. The ideal case to start with is a mitral valve replacement or a straight forward mitral valve repair without pulmonary hypertension in a non-obese patient with good left ventricular function, no mitral calcification, no chest deformity, no previous cardiac surgery and no adhesions in the right chest. The most reproducible and simplest technique is access through right mini thoracotomy (totally thoracoscopic or with video assistance and direct view), cardiopulmonary bypass through peripheral femoral arterial and venous cannulation and aortic occlusion with a direct transthoracic clamp. In a more experienced stage, more complex patients can be operated on with more complex techniques, such as the use of the endo-aortic balloon, robotic assistance and patient specific modified cannulation configurations. However, moving towards more complex techniques should never compromise operative safety and reparability of the mitral valve. The number required to complete the learning curve is currently reported at 75 – 125 cases and at least 2 cases per week to maintain optimal surgical outcome. Technology (such as a training simulator) can decrease this number, but is not yet confirmed by studies.[13][14]
This entry is adapted from the peer-reviewed paper 10.3390/jcm11205993
References
- Carpentier, A; Loulmet, D; Le Bret, E e.a Open heart operation under videosurgery and minithoracotomie. First case (mitral valvuloplasty) operated with succes. C.R. Acad. Sci. 1996, 319, 219-223.
- W.Randolph Chitwood; Joseph R. Elbeery; William H.H. Chapman; Jon M. Moran; Robert L. Lust; William A. Wooden; David H. Deaton; Video-assisted minimally invasive mitral valve surgery: The “micro-mitral” operation. The Journal of Thoracic and Cardiovascular Surgery 1997, 113, 413-414, 10.1016/s0022-5223(97)70341-6.
- F.W. Mohr; V. Falk; A. Diegeler; T. Walther; J.A.M. van Son; R. Autschbach; Hans G. Borst; Minimally Invasive Port-Access Mitral Valve Surgery. The Journal of Thoracic and Cardiovascular Surgery 1998, 115, 567-576, 10.1016/s0022-5223(98)70320-4.
- Piroze M. Davierwala; Joerg Seeburger; Bettina Pfannmueller; Jens Garbade; Martin Misfeld; Michael A. Borger; Friedrich W. Mohr; Minimally invasive mitral valve surgery: “The Leipzig experience”. Annals of Cardiothoracic Surgery 2013, 2, 744-750, 10.3978/j.issn.2225-319X.2013.10.14.
- Mattia Glauber; Antonio Miceli; Daniele Canarutto; Antonio Lio; Michele Murzi; Daniyar Gilmanov; Matteo Ferrarini; Pier Andrea Farneti; Eugenio L. Quaini; Marco Solinas; et al. Early and long-term outcomes of minimally invasive mitral valve surgery through right minithoracotomy: a 10-year experience in 1604 patients. Journal of Cardiothoracic Surgery 2015, 10, 1-9, 10.1186/s13019-015-0390-y.
- Kinsing Ko; Thom L De Kroon; Marco C Post; Johannes C Kelder; Karen F Schut; Nabil Saouti; Bart P Van Putte; Minimally invasive mitral valve surgery: a systematic safety analysis. Open Heart 2020, 7, e001393, 10.1136/openhrt-2020-001393.
- Kinsing Ko; Thom L. de Kroon; Johannes C. Kelder; Nabil Saouti; Bart P. van Putte; Reoperative Mitral Valve Surgery Through Port Access. Seminars in Thoracic and Cardiovascular Surgery 2021, Online ahead of print., 0, 10.1053/j.semtcvs.2021.08.014.
- Joerg Seeburger; Michael A. Borger; Volkmar Falk; Jurgen Passage; Thomas Walther; Nicolas Doll; Friedrich W. Mohr; Minimally Invasive Mitral Valve Surgery After Previous Sternotomy: Experience in 181 Patients. The Annals of Thoracic Surgery 2009, 87, 709-714, 10.1016/j.athoracsur.2008.11.053.
- Michele Murzi; Antonio Miceli; Gioia Di Stefano; Alfredo G. Cerillo; Pierandrea Farneti; Marco Solinas; Mattia Glauber; Minimally invasive right thoracotomy approach for mitral valve surgery in patients with previous sternotomy: A single institution experience with 173 patients. The Journal of Thoracic and Cardiovascular Surgery 2014, 148, 2763-2768, 10.1016/j.jtcvs.2014.07.108.
- Matthew A. Romano; Jonathan W. Haft; Francis D. Pagani; Steven F. Bolling; Beating heart surgery via right thoracotomy for reoperative mitral valve surgery: A safe and effective operative alternative. The Journal of Thoracic and Cardiovascular Surgery 2012, 144, 334-339, 10.1016/j.jtcvs.2011.09.026.
- Alexander P. Nissen; Charles C. Miller; Vinod H. Thourani; Y. Joseph Woo; James S. Gammie; Gorav Ailawadi; Tom C. Nguyen; Less Invasive Mitral Surgery Versus Conventional Sternotomy Stratified by Mitral Pathology. The Annals of Thoracic Surgery 2020, 111, 819-827, 10.1016/j.athoracsur.2020.05.145.
- David M. Holzhey; Joerg Seeburger; Martin Misfeld; Michael A. Borger; Friedrich W. Mohr; Learning Minimally Invasive Mitral Valve Surgery. Circulation 2013, 128, 483-491, 10.1161/circulationaha.112.001402.
- Mikael Kastengren; Peter Svenarud; Göran Källner; Anders Franco-Cereceda; Jan Liska; Isak Gran; Magnus Dalén; Minimally invasive versus sternotomy mitral valve surgery when initiating a minimally invasive programme. European Journal of Cardio-Thoracic Surgery 2020, 58, 1168-1174, 10.1093/ejcts/ezaa232.
- Gammie JS, Zhao Y, Peterson ED e.a; Less-invasive mitreal valve operations; trends en oputcomes from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg 2010;90(5): 1401-8, 10 el; discussion 8-10.
