Worldwide, 50 million people suffer from dementia, a group of symptoms affecting cognitive and social functions, progressing severely enough to interfere with daily life. Alzheimer’s disease (AD) accounts for most of the dementia cases. Pathological and clinical findings have led to proposing several hypotheses of AD pathogenesis, finding a presence of positive feedback loops and additionally observing the disturbance of a branch of tryptophan metabolism, the kynurenine (KYN) pathway. Either causative or resultant of dementia, elevated levels of neurotoxic KYN metabolites are observed, potentially upregulating multiple feedback loops of AD pathogenesis. Memantine is an N-methyl-D-aspartate glutamatergic receptor (NMDAR) antagonist, which belongs to one of only two classes of medications approved for clinical use, but other NMDAR modulators have been explored so far in vain. An endogenous KYN pathway metabolite, kynurenic acid (KYNA), likewise inhibits the excitotoxic NMDAR. Besides its anti-excitotoxicity, KYNA is a multitarget compound that triggers anti-inflammatory and antioxidant activities. Modifying the KYNA level is a potential multitarget strategy to normalize the disturbed KYN pathway and thus to alleviate juxtaposing AD pathogeneses.
1. Multiple Positive Feedback Loops via Kynurenine Metabolites
Dementia patients have been associated with the disturbance of tryptophan (TRP) metabolism and its downward catabolic branch, the KYN pathway. Low circulating TRP levels, elevated neurotoxic KYN metabolites, and a reduced neuroprotective KYN metabolite are observed in elderly patients with neurodegenerative disease such as AD, PD, and HD [
36]. Either causative or resultants of AD pathogenesis, the aberrant KYN pathway lies not only in a close connection with AD pathophysiology but also may play a critical role in potentiating the multiple positive feedback loops of AD pathology.
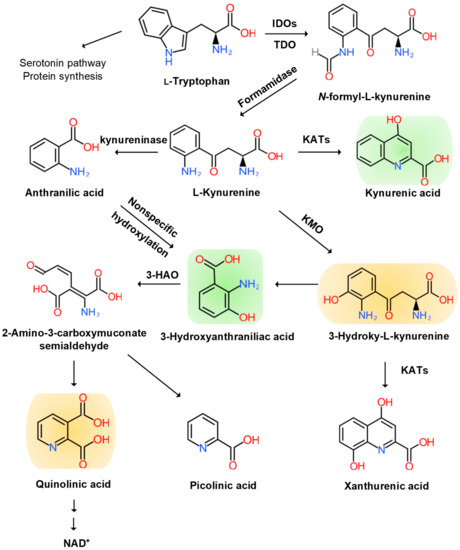
The KYN pathway transforms over 95% of TRP into a series of small bioactive molecules with neurotoxic, neuroprotective, oxidative, or antioxidative properties. Inflammation activates several key enzymes in the pathway [
37]. The indole ring of TRP is oxidized to produce N-formyl KYN by the TRP dioxygenase (TDO) in the liver, the indolamine-2,3-dioxygenase (IDO) 1 in the brain, and peripheral tissues and IDO 2 in the liver, kidney, and antigen-presenting cells [
38]. TDO is activated by the glucocorticoid stress hormone, cortisol; IDO1 is activated by the pro-inflammatory cytokines, interferon (IFN)-α, IL-1β, IFN-γ, and TNF-α, and it is inhibited by the anti-inflammatory cytokines, IL-2, IL-4, IL-10, and transforming growth factor-β (TGF-β) through IFN-γ. IDO2 knockout mouse (IDO2
−/− mice) revealed that IDO2 has a pro-inflammatory role and contributes to autoantibody production [
39]. Thus, stressful events and inflammatory responses activate the rate-limiting TRP enzymes to cascade down in the KYN pathway.
N-formyl KYN is converted by formamidase to L-KYN, which is a substrate of three downstream metabolites: anthranilic acid (AA) by kynureninase, 3-hydroxy-KYN (3-HK) by KYN-3-monooxygenase (KMO), and KYNA by pyridoxal 5′-phosphate (PLP)-dependent KYN aminotransferases (KATs) [
40]. AA and its metabolite, 3-hydroxy-AA (3-HAA), are found to suppress pro-inflammatory cytokine IFN-γ, T and B lymphocyte cell proliferation, and Th1 cell activity and invoke anti-inflammatory cytokine, IL-10 [
41]. 3-HK generates highly reactive free radicals. An elevation of 3-HK levels has been shown to be related to excitotoxic injury and is observed in patients with neurodegenerative diseases [
42].
A KAT isoform, KAT II, functions in the physiological pH range and may be responsible for most of the KYNA synthesis in the brain. KATs also convert 3-HK to xanthurenic acid (XA) [
43]. KYNA is an antagonist at ionotropic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), NMDA, kainate glutamate receptors, and the α7 nicotinic Ach receptor [
44]. However, the role of KYNA at the α7 nicotinic Ach receptor remains controversial [
45]. KYNA binds to the G protein-coupled receptor (GPR) 35 (GPR35) expressed in glia, macrophages, and monocytes to reduce glutamate release in brain and pro-inflammatory cytokine release in cell lines. KYNA also binds to aryl hydrocarbon receptor (AhR) to alleviate adaptive immune responses [
46].
3-HK and AA are converted by 3-hydroxyanthranilate oxidase to highly redox-active 3-HAA, which may play a role in the regulation of oxidative stress. 3-HAA suppresses cytokine and chemokine production and neurotoxicity induced by IL-1 or IFN-γ [
47]. 3-HAA is converted by 3-hydroxyanthranilate dioxygenase to 2-amino-3-carboxymuconate semialdehyde, which is further transformed into picolinic acid (PIC) and an excitotoxic and free-radical metabolite, quinolinic acid (QUIN). The pro-inflammatory cytokine IFN-γ stimulates IDO, formamidase, and kynurenine-3-monooxygenase (KMO) activities in human microglia and macrophages, leading to increased QUIN synthesis. The activation of macrophages and glial cells induces the increased production of QUIN [
48]. Anti-inflammatory steroid agents such as dexamethasone suppress QUIN concentrations in the brain following immune stimulation [
49]. Finally, QUIN is metabolized in subsequent steps into nicotinic acid dinucleotide (NADH) (
Figure 2).
Figure 2. Kynurenine Branch of Tryptophan Metabolism. More than 95% of tryptophan is metabolized in the kynurenine (KYN) pathway except for serotonin metabolism and protein synthesis. Tryptophan (TRP) is converted to KYN by the hepatic rate-limiting tryptophan 2,3-dioxygenase (TDO) and ubiquitous rate-limiting indoleamine 2, 3-oxygenase (IDO) 1, each of which is induced by cortisol, and interferon (IFN)-α, IFN-γ, and tumor necrosis factor (TNF)-α, respectively. KYN is converted to anthranilic acid (AA) by the kynureninase, 3-hydroxy-L-kynurenine (3-HK) is converted by the KYN-3-monooxygenase (KMO), and kynurenic acid (KYNA) is converted by KYN aminotransferases (KATs). KYNA is an antagonist at the NMDA receptor. AA and 3-HK are converted to 3-hydroxyanthranillic acid (3-HAA) and further to picolinic acid (PIC) and quinolinic acid (QUIN). 3-HK and QUIN are agonists at the NMDA receptor. QUIN is converted to nicotinamide adenine dinucleotide (NAD+), which is a feedback inhibitor of TDO. Neurotoxic KYNs are shown in orange, and neuromodulartory KYNs are shown in green.
2. Kynurenines in Major Neurocognitive Disorders
A systematic review was conducted on the status of KYNs in major NCD. A total of 30,004 articles matched our database search. Out of 586 articles 10 meta-analysis and systematic reviews, a total of 212 articles were assessed for eligibility. Finally, 23 articles were deemed for synthesis in this systematic review. The methodological quality and risk of bias assessment are shown in Table 1. Evidence levels of neurotoxic and neuromodulatory KYN levels were assessed at low risk of bias for MDD; high risk of bias for AD, PD, and HD; unclear of bias for VCD, bipolar disorder (BP), generalized anxiety disorder (GAD), and autism spectrum disorders (ASD) (Table 1).
Table 1. Studies included for systematic review synthesis, study designs, and risk bias assessment.
| Diseases |
Study Types |
Reference Numbers
or Sample Numbers
(Disease/Control) |
Samples |
Risk of Bias |
| Neurodegenerative diseases |
| Alzheimer’s disease [50,51,52,53,54] |
| Guillemin et al., 2005 [51] |
case-control study |
6/4 |
brain tissue |
High risk |
| Bonda et al., 2010 [52] |
case-control study |
12/7 |
brain tissue |
| Gulaj et al., 2010 [53] |
case-control study |
34/18 |
serum |
| Schwarcz et al., 2013 [54] |
case-control study |
20/19 |
serum |
| Parkinson’s disease [55,56,57,58,59] |
| Hartai et al., 2005 [57] |
case-control study |
19/17 |
plasma, RBC |
High risk |
| Lewitt et al., 2013 [58] |
case-control study |
48/57 |
CSF |
| Chang et al., 2018 [59] |
case-control study |
118/37 |
plasma |
| Huntington’s disease [60,61,62,63,64] |
| Reynolds and Pearson, 1989 [64] |
case-control study |
12/11 |
postmortem brain tissue |
High risk |
| Beal et al., 1992 [62] |
case-control study |
14–30/25–40 |
postmortem brain tissue |
| Jauch et al., 1995 [63] |
case-control study |
17/17 |
postmortem brain tissue |
| Stoy et al., 2005 [61] |
case-control study |
15/11 |
plasma |
| Vascular Cognitive Dementia |
|
|
|
Unclear |
| Darlington et al., 2007 [65] |
case-control study |
50/35 |
serum |
| Yan et al., 2015 [66] |
case-control study |
28/20,11 |
serum, CSF |
| Psychiatric disorders |
| Major depressive disorder |
|
|
|
Low risk |
| Ogawa et al., 2014 [67] |
meta-analysis |
10 |
plasma |
| Réus et al., 2015 [68] |
systematic review |
29 |
plasma, blood, serum,
CSF, urine, brain tissue |
| Ogyu et al., 2018 [69] |
meta-analysis |
22 |
plasma |
| Bipolar disorder [70,71,72,73,74,75,76,77] |
| Birner et al., 2017 [75] |
case-control study |
143/101 |
blood |
Unclear |
| Wang et al., 2018 [76] |
meta-analysis |
16 |
CSF |
| Arnone et al., 2018 [77] |
meta-analysis |
5 |
serum |
| Generalized anxiety disorder |
| Orlikov et al., 1994 [78] |
case-control study |
16/15 |
plasma |
Unclear |
| Altmaier et al., 2013 [79] |
case-control study |
386/116 |
serum |
| Autism spectrum disorder |
| Lim et al., 2016 [80] |
case-control study |
15/12 |
blood |
Unclear |
| Bryn et al., 2017 [81] |
case-control study |
30/30 |
serum |
2.1. Kynurenines in Neuodegenerative Diseases
Increased KYN, KYNA, and QUIN in serum and CSF were associated with aging [
50]. Altered levels of KYN metabolites have been observed in patients with AD, PD, HD, and VCD. An increased KYN/TRP ratio of the plasma and CSF, increased levels of IDO in the brain, and immunoreactivity for both IDO and QUIN in the microglia, astrocytes, and neurons of hippocampal tissue were observed in AD [
51,
52,
53,
54]. It has been suggested that KYNs are involved in the regulation of glutamate neurotransmission, neuroprotection, and immune responses in AD. Furthermore, an increased CSF 3-HK/KYN ratio was correlated with t-tau and p-tau, while plasma KYN and PIC inversely correlated with p-tau and t-tau, respectively [
55]. KYNA levels are decreased in the plasma, witnessing the shift toward neurotoxic metabolites over neuroprotective ones in AD [
53]. Higher and lower levels of KYN were associated with a higher Neuropsychiatric Inventory (NPI) total score, and a lower KYN/KYNA ratio indicated risk for hallucination in AD and Lewy bodies dementia [
56].
The plasma samples of PD patients showed significant lower activities of KAT I and KAT II with a decreasing tendency of plasma KYNA levels [
57]. A metabolomic profiling study of CSF from PD patients showed increased 3-HK levels [
58]. A metabolomic evaluation showed that a lower KYNA/KYN ratio, higher QUIN level, and higher QUIN/KYNA ratio were observed in the plasma of PD patients, suggesting a shift toward neurotoxic QUIN and away from neuroprotective KYNA synthesis [
59]. The alterations in KYN metabolite levels may contribute to pathogenesis in PD, and the KYN pathway intervention was proposed to alleviate PD symptoms through neuroprotection [
60].
The KYN/TRP ratio was higher, while the KYNA/KYN ratio was lower in the plasma of HD patients than controls [
61]. A postmortem brain study showed decreased KYN levels in the middle and inferior cortex, decreased KYNA levels in the precentral gyrus, frontal, and temporal cortex, and decreased 3-HK levels in the inferior temporal cortex [
62]. Another study also showed decreased KYNA levels in the caudate nucleus and lower KAT I and KAT II in the putamen of HD patients [
63]. However, 3-HK levels were significantly higher in the frontal and temporal cortex in HD brain samples [
64]. A significant reduction in TRP levels was found at several days after stroke onset, and the KYN/TRP ratio was elevated much higher in stroke patients [
65]. KYNA levels were higher in patients who died within 21 days after stroke [
66].
Many studies have presented disturbance of KYN metabolism in patients with dementia. Increased levels of neurotoxic KYNs were observed in AD, PD, HD, and VCD. It is intriguing that levels of neuroprotective KYNA were decreased in AD, PD, and HD, but increased in VCD. Further study is expected to uncover the status and change of neurotoxic and neuroprotective KYN metabolites under progression of the diseases (Table 2).
Table 2. Systematic synthesis of kynurenine levels in neurodegenerative diseases and psychiatric disorders. ↑: increase; ↓: decrease; ?: unclear or unknown.
2.2. Kynurenines in Psychiatric Disorders
Cognitive domains are also affected in psychiatric disorders such as major depressive disorder (MDD), bipolar disorder (BD), generalized anxiety disorder (GAD), and autism spectrum disorders (ASD). Lower levels of plasma TRP, KYN, and KYNA were observed in MDD. A higher level of QUIN immunoreactivity was detected in the prefrontal cortex and hippocampus of the postmortem samples of MDD patients [
67,
68,
69]. Chronic stress has been linked in MDD to structural brain damages including a loss of dendritic spines and synapses, reduced dendritic arborization, and diminished glial cells in the hippocampus [
70]. A possible relationship between KYN metabolism and suicide ideation has been investigated in psychiatric patients, including non-MDD patients. Higher levels of CSF QUIN, a higher ratio of CSF QUIN/KYNA, and lower levels of CSF KYNA have been associated with suicide attempts in psychiatric patients [
71]. Lower levels of PIC, lower ratio of PIC/QUIN, and a higher ratio of KYN/TRP were reported in patients with suicide attempts. However, studies have not reached a consensus on the upregulation or downregulation of TDO/IDO enzymes among the suicide-prone population [
72].
Cognitive deficits of verbal/visual memory and executive tasks have been observed during depressive episodes in BD, while executive dysfunction and attention deficits have been reported during manic episodes in BD [
73,
74]. A case-control study reported increased 3-HK/KYN and 3-HK/KYNA ratio and decreased KYNA levels in BD [
75]. A meta-analysis reported an increased level of KYNA in the CSF of bipolar patients [
76]. However, another meta-analysis reported no significant difference of TRP and KYN levels, nor KYN/TRP and KYNA/QUIN ratios in serum from BD patients [
77]. Further intensive study is expected on the status of the KYN metabolites in manic and depressive phases of BD patients. In patients with GAD, decreased levels of plasma KYN were observed in endogenous anxiety and normalized after treatment [
78]. Significantly lower levels of KYN have been associated with Type D personality, which has been characterized by negative affectivity and social inhibition [
79].
The status of KYN metabolites has not reached a consensus in ASD. The blood KYN and QUIN levels and KYN/TRP ratio were found significantly higher, PIC levels were significantly lower, and KYNA levels were unchanged in ASD [
80]. The serum KYNA level was significantly lower, while the KYN/KYNA ratio was significantly higher in children with ASD [
81]. The results have not reached consensus, which is most probably due to a small number of studies and the heterogenous etiologies of ASD (
Table 2).
It is intriguing that lower levels of KYNA is associated with psychiatric disorders affecting cognitive domains, but higher levels of KYNA is observed in patients suffering from schizophrenia, which barely exhibits cognitive symptoms [
76,
82]. Further investigation is expected on the relationship between KYN metabolism and psychiatric disorders. Disturbance of TRP and KYN metabolisms has been observed in patients suffering from major NCD and is found to be closely linked to AD pathogenesis and dementia in which multiple positive feedback loops through an imbalance of KYN metabolites may potentially contribute to the exacerbation of dementia (
Figure 3).
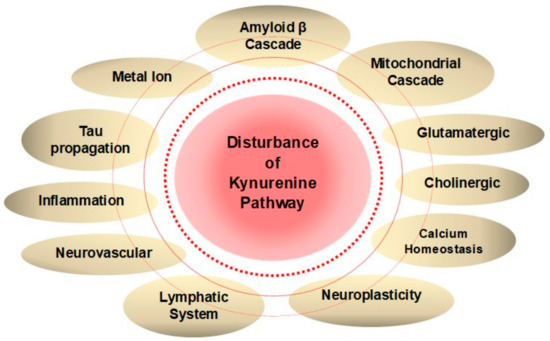
Figure 3. Disturbance of Kynurenine Metabolism Wires Multiple Positive Feedback Loops of Alzheimer’s Disease. Hypotheses of Alzheimer’s disease (AD) pathogenesis derived from anatomical, clinical, and medicinal findings are closely connected to each other, and many positive feedback loops exist to exacerbate the disease. Disturbance of a branch of tryptophan metabolism, kynurenine (KYN) pathway lies in a close connection with various pathogeneses of dementia. Increased neurotoxic KYN metabolites and decreased neuroprotective kynurenic acid (KYNA) may potentiate multiple feedback loops of AD pathogenesis.
This entry is adapted from the peer-reviewed paper 10.3390/molecules25030564